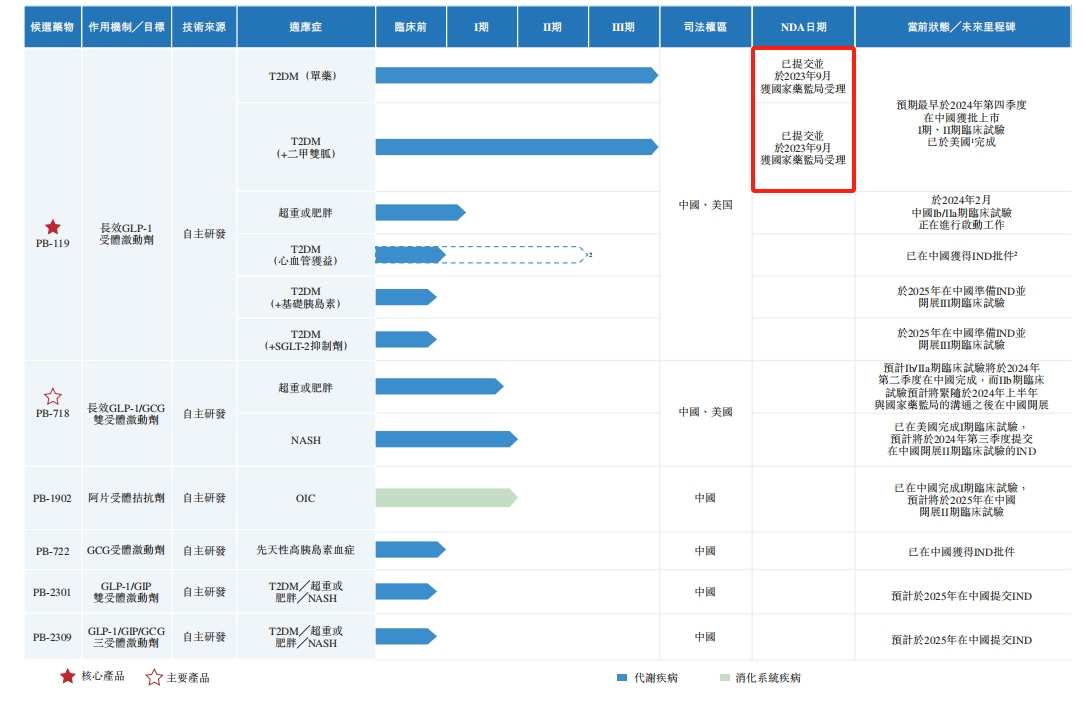
① Peger Biotech has one GLP-1 product, PB-119, which is in the NDA stage, and five other products under clinical and pre-clinical development; ② Since the core product has not yet been approved, Peger Biotech is still in a “three no” state with “no products, no revenue, no profit”.
“Science and Technology Innovation Board Daily”, March 5 (Reporter Zheng Bingxun) “Peger Biopharmaceuticals (Suzhou) Co., Ltd.” (hereinafter referred to as “Peger Biotech”) recently restarted the IPO process, but the destination was changed to the Hong Kong Stock Exchange.
According to the IPO documents, Peger Biotech is a biotechnology company focusing on independent research and development of innovative treatments for chronic diseases, focusing on the field of metabolic disorders.
 Currently, Peger Biotech has a GLP-1 product PB-119 in the NDA stage, and five other clinical-stage and pre-clinical research products. The main indications cover common chronic diseases and metabolic diseases such as type 2 diabetes (T2DM), obesity, non-alcoholic steatohepatitis (“NASH”), constipation caused by opioids (“OIC”), and congenital hyperinsulinemia.
Currently, Peger Biotech has a GLP-1 product PB-119 in the NDA stage, and five other clinical-stage and pre-clinical research products. The main indications cover common chronic diseases and metabolic diseases such as type 2 diabetes (T2DM), obesity, non-alcoholic steatohepatitis (“NASH”), constipation caused by opioids (“OIC”), and congenital hyperinsulinemia.
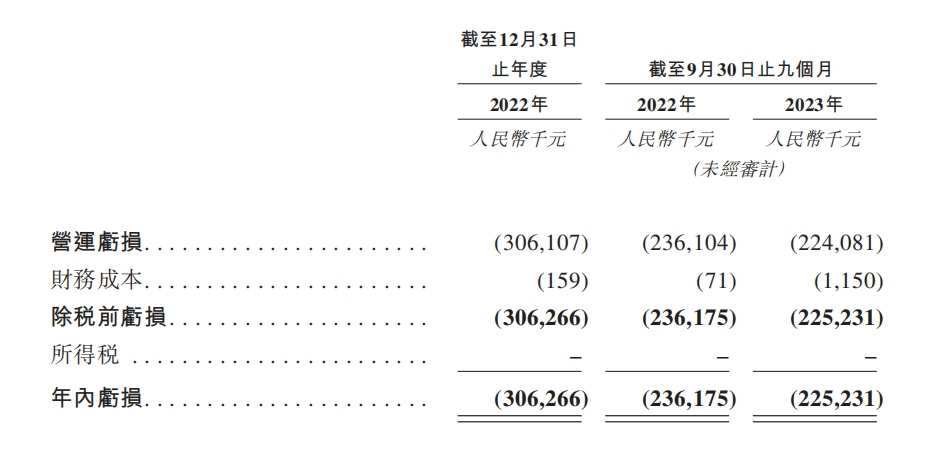
Since the core product has not yet been approved, Peger Biotech is still in a “three no” state with “no products, no revenue, no profit”. In 2022 and the first nine months of 2023, Peger Biotech invested 280 million yuan and 193 million yuan respectively. In the same period, losses amounted to 306 million yuan and 225 million yuan.
▌Bet on GLP-1 products in the NDA phase
In response to an inquiry from the Shanghai Stock Exchange, Peger Biotech once quoted Frost & Sullivan's report that the global diabetes drug market was close to 70 billion US dollars in 2020, expected to exceed 90 billion US dollars in 5 years, and further grow to close to 110 billion US dollars in 2030. In the same period, the Chinese market also grew rapidly. The market size of diabetes drugs in China was 63.2 billion yuan in 2020, and is expected to grow to 170 billion yuan in 2030.
In addition, according to Novo Nordisk's report, GLP-1 receptor agonists accounted for 18.8% of global diabetes drugs in 2020, with a market share of more than US$13.1 billion. In contrast, GLP-1 accounts for only 2.6% of diabetes drugs in China, with a market share of about 1.6 billion yuan.
In recent years, GLP-1 drugs can effectively reduce blood sugar while improving body weight and reducing cardiovascular risk, and their therapeutic status in domestic and foreign guidelines has continued to improve. Precisely because it sees the potential of GLP-1 products, Peger Biotech focused its future development on the core product PB-119.
PB-119 is a long-acting GLP-1 receptor agonist developed by Peger Biotech. It is mainly used in first-line treatment of T2DM and obesity. Using the core technology polyethylene glycol (PEG) conversion technology, Peger Biotech extended the half-life of PB-119 and achieved weekly administration.
In September 2023, the State Drug Administration accepted a new drug application (NDA) for PB-119 as a single drug and metformin for the treatment of T2DM. As a result, PB-119 became the fastest progressing clinical-stage long-term GLP-1 receptor agonist in China. Peger Biotech expects that PB-119 will be approved for domestic listing as early as Q4 2024 and commercialized in 2025. At the same time, PB-119 also completed phase II clinical trials of T2DM in the US, preparing ahead of time to expand overseas markets.
However, the PB-119 is still unlisted and is already facing numerous competitors. By the end of September 2023, there were more than 15 domestic competitors for PB-119 for the treatment of T2DM. In the same period, there were more than 20 competitors outside of China.
Peger Biotech also clearly realizes that the company's business, financial situation, operating performance and prospects for the next few years will largely depend on the successful approval and sale of PB-119. “If we are unable to successfully obtain regulatory approval, commercialize, or complete clinical research and development to expand the indications of PB-119... our business, financial situation, operating performance and prospects may be significantly adversely affected.” Peger Biotech clearly stated in the IPO documents.
Peggy's concerns are not without reason. Currently, with the exception of PB-119, which is expected to be approved for listing to generate revenue, Peger Biotech's other 5 products under development cannot be expected for an hour and a half.
Among them, research on PB-718 to treat overweight or obesity and NASH is in clinical phase I; research on PB-1902 to treat OIC is also in clinical phase I, and phase II clinical trials are expected to be carried out only in 2025; the IND application for PB-772 was only approved in May 2023. The other two products, PB-2301 and PB-2309, are only in the pre-clinical stage.
It is worth mentioning that when no income is added to the account, the cash on Peger Bio's account is also being rapidly consumed. As of the end of September 2022, Peger Biotech's cash and cash equivalents were $301 million. One year later, by the end of September 2023, this amount was reduced to $103 million.
▌A total of 1.3 billion yuan has been raised, and shareholders such as Qianhai Fund, Tiger Pharmaceuticals, and Tianshili stand behind it
In fact, since its establishment, Peger Biotech has completed 9 rounds of financing, raising a total amount of about 50.3 million US dollars and 1.05 billion yuan, and has gradually introduced a number of important investors including but not limited to Mingly (Mingli China Growth Fund), Kaifeng Ventures, True Wing, Tianshi (Hong Kong), Tiger Pharmaceuticals, etc.
Among them, the higher-amount financing round included the E round of 21 million US dollars and the F round of 783 million yuan. With the completion of the F+ round financing in June 2023, Peger Biotech's valuation reached 4 billion yuan.

Judging from the time of establishment, the earliest history of Peger Biotech can be traced back to July 2001. It was founded by the former holding company Pan Asia. Pan Asia's authorized share capital is 50,000 US dollars, and founder Michael Min Xu (Michael Min Xu) holds 50% of the shares. In May 2008, Peger Biotech was established as a limited company with a registered capital of 500,000 US dollars. It is still wholly owned by Pan Asia. At this time, Pan Asia owned 67.86% of the shares. In December 2020, Peger Biotech was officially restructured as a limited company and changed its name to its current name, with a registered capital of 355 million yuan.
After the share transfer was completed, Peger Biotech completed a series of investments. As of the last practical date, Peger Biotech's registered capital was RMB 367 million. The number of shareholders exceeds 60. Xu Min is the largest shareholder, holding 58.0819 million shares, with a shareholding ratio of 15.84%. The single largest shareholder group consists of Xu Min, Xiangjun Zhou, Xu Yuhong, and Shanghai Su Jie, holding a total of 27.37% of the shares. The parties signed a concerted action agreement in 2021.

After equity penetration, it can be discovered that there are several important shareholders standing behind Peger Biotech.
Among them, Mingly holds 9.50% of Peger Biotech's shares, and the total assets managed by its fund management team exceed HK$1 billion, and invests in biomedical companies such as Rongchang Pharmaceuticals (688331.SH, 9995.HK). Second, Qianhai Fund holds 2.26% of Peger Biotech's shares. Since 2015, Qianhai Fund has invested in the biopharmaceutical sector and has assets under management of about 24 billion yuan. Of these, 48 portfolio companies are in the biopharmaceutical sector, including Kangfang Biotech (9926.HK), Geli Pharmaceuticals (1672.HK), and Pumen Technology (688389.SH).
In addition to this, Peger Biotech also has a number of pharmaceutical companies behind it. Among them, Tasley Hong Kong, which holds 3.47% of the shares, is a wholly-owned subsidiary of Tianshili Pharmaceuticals (600535.SH), which lays out an innovative drug circuit and has a leading position in the industry. Meanwhile, Hangzhou Tiger, which holds 99.98% of Tiger Pharmaceuticals (3347.HK), owns 2.63% of Peger Biotech's shares, and Hong Kong Tiger Pharmtech, which is wholly-owned, also holds 1.16% of Peger Biotech's shares.
Currently, Peger Biotech's board of directors consists of 9 directors, including 2 executive directors, 4 non-executive directors, and 3 independent non-executive directors. Xu Min is the chairman, executive director and general manager of the company.
Xu Min, 59, is responsible for Peger Biotech's overall strategic planning, business direction and operation management. He received a bachelor's degree in medicine from Hunan Medical University in China (now Xiangya Medical School of Central South University) and a doctorate degree in biophysics from Columbia University in the US, and has published 6 collaborative articles in journals such as “Science”. Xu Min has over 30 years of working and management experience in the healthcare industry. Previously, he also served as an independent non-executive director of Pioneer Pharmaceutical (9939.HK).

 目前,派格生物拥有一款处于NDA阶段的GLP-1产品PB-119,以及另外五款处于临床阶段及临床前的在研产品,主要适应症覆盖2型糖尿病(T2DM)、肥胖症、非酒精性脂肪性肝炎(“NASH”)、阿片类药物引起的便秘(“OIC”)及先天性高胰岛素血症等常见慢病及代谢疾病。
目前,派格生物拥有一款处于NDA阶段的GLP-1产品PB-119,以及另外五款处于临床阶段及临床前的在研产品,主要适应症覆盖2型糖尿病(T2DM)、肥胖症、非酒精性脂肪性肝炎(“NASH”)、阿片类药物引起的便秘(“OIC”)及先天性高胰岛素血症等常见慢病及代谢疾病。