已於2024年11月1日向美國證券交易委員會提交。
登記號333-_
美國
證券交易委員會
華盛頓特區,20549
表格S-1
註冊聲明
在……下面
1933年《證券法》
Autonomix MEDICAL,Inc.
(註冊人的確切姓名載於其章程)
|
特拉華州 |
3841 |
47-1607810 |
|
(述明或其他司法管轄權 公司或組織) |
(主要標準工業 分類代碼編號) |
(稅務局僱主 識別碼) |
水道大道21號,300套房
德克薩斯州伍德蘭77380
(713) 588-6150
(註冊人的地址,包括郵政編碼和電話號碼,包括地區代碼’s主要行政辦公室)
布拉德·豪瑟
首席執行官
水道大道21號,300套房
德克薩斯州伍德蘭77380
(713) 588-6150
(服務代理的名稱、地址,包括郵政編碼和電話號碼,包括區號)
複製到:
|
卡瓦斯·S·帕夫裏 喬納森·鄧肯 ArentFox Schiff LLP K街西北1717號 華盛頓特區,郵編:20006 電話:(202)724-6847 傳真:(202)778-6460 |
萊斯利·馬洛,Esq. 帕特里克·伊根先生 空白羅馬有限責任公司 美洲大道1271號 紐約州紐約市,郵編:10020 電話:(212)885-5000 傳真:(212)885-5001 |
建議向公衆出售的大約開始日期: 在本註冊聲明生效日期後儘快。
如果根據1933年證券法第415條,在此表格上登記的任何證券將延遲或連續發售,請勾選以下方框。☒
如果本表格是根據證券法第462(B)條登記發行的額外證券而提交的,請勾選以下方框,並列出同一發售的較早生效登記聲明的證券法登記聲明編號。☐
如果此表格是根據證券法下的規則462(C)提交的生效後修訂,請勾選以下框並列出相同發售的較早生效註冊聲明的證券法註冊聲明編號。☐
如果此表格是根據證券法下的規則462(D)提交的生效後修訂,請勾選以下框並列出相同發售的較早生效註冊聲明的證券法註冊聲明編號。☐
用複選標記表示註冊人是大型加速申報公司、加速申報公司、非加速申報公司、較小的報告公司或新興成長型公司。請參閱《交易法》第12b-2條規則中「大型加速申報公司」、「加速申報公司」、「較小申報公司」和「新興成長型公司」的定義:
|
大型加速文件夾 |
☐ |
加速文件管理器 |
☐ |
|
非加速文件服務器 |
☒ |
小型上市公司 |
☒ |
|
新興成長型公司 |
☒ |
如果是一家新興成長型公司,用複選標記表示註冊人是否已選擇不使用延長的過渡期來遵守根據證券法第7(A)(2)(B)節提供的任何新的或修訂的財務會計準則。☐
註冊人特此在可能需要的日期或多個日期對本註冊聲明進行修改,以推遲其生效日期,直到註冊人提交進一步的修改,其中明確指出本註冊聲明此後將根據第條生效 1933年證券法第8(a)條或直至登記聲明於委員會根據上述部分行事的日期生效 8(a),可以確定。
這份初步招股說明書中的信息不完整,可能會被更改。在提交給美國證券交易委員會的註冊聲明生效之前,這些證券不得出售。這份初步招股說明書不是出售這些證券的要約,我們也不會在任何不允許要約或出售的司法管轄區徵求購買這些證券的要約。
|
初步招股說明書 |
有待完成 |
日期:2024年11月1日 |

多達698,812個普通股單位
每個普通股單位由一股普通股和一份購買一股普通股的A系列許可證組成
A系列認股權行使後可發行的普通股最多698,812股
多達698,812個PFW單位
每個PFW單位由一份用於購買一股普通股的預先融資憑證和一份用於購買一股普通股的A系列憑證組成
預融資憑證行使後可發行的最多698,812股普通股
A系列令下的普通股最多698,812股
購買最多41,928股普通股的代表性令狀
代表性認股權行使後可發行的最多41,928股普通股
這是特拉華州一家公司Autonomix Medical,Inc.的698,812股普通股單位(「普通股單位」)的堅定承諾公開發行,每個普通股單位包括:(I)一股我們的普通股,每股票面價值0.001美元(「股份」,「普通股」或「普通股」),以及(Ii)購買一股我們普通股的A系列認股權證(「A系列認股權證」或「普通股認股權證」)。每個普通股單位的假定公開發行價爲14.31美元,基於上一次報告的我們普通股的銷售價格,即2024年10月31日在納斯達克資本市場(以下簡稱納斯達克)上報告的價格。普通權證的假定初始行使價格爲每股_。普通權證可立即行使,但須受本文所述的某些限制所規限,並自本次發售結束之日起計滿五年。我們還提供我們普通股的股票,這些股票在普通權證行使後可以不時發行。
我們還向每一位在本次發售中購買普通股單位的購買者提供機會,如果購買者在本次發售中購買普通股單位,將導致購買者連同其關聯公司和某些關聯方在本次發售完成後立即實益擁有超過4.99%(或在購買者選擇時,9.99%)我們的已發行普通股,如果購買者選擇,有機會購買最多698,812美元的預融資認股權證單位(「PFW單位」,與普通股單位一起,「單位」)。以代替普通股,否則將導致購買者的實益所有權超過我們已發行普通股的4.99%(或在購買者選擇時,超過9.99%)。每個PFW單位包括:(I)購買一股我們普通股的預融資認股權證(「預融資權證」),以及(Ii)購買一股我們普通股的A系列認股權證。包括在PFW單位中的A系列權證與包括在普通股單位中的A系列權證相同。除有限的例外情況外,如果預先出資認股權證持有人及其關聯公司在行使該權利後將實益擁有超過4.99%(或經持有人選擇,超過9.99%)的已發行普通股數量,則預先出資認股權證持有人將無權行使其預先出資認股權證的任何部分。每一份預先出資的認股權證將可以一股普通股的價格行使,行使價格爲每股普通股0.001美元。每個PFW單位的公開發行價等於每個普通股單位的公開發行價減去0.001美元。每份預付資金認股權證在發行時即可行使,並在全部行使時失效。我們還提供我們普通股的股票,這些股票在行使預先出資的認股權證和與之相關的普通股認股權證後可以不時發行。對於我們出售的每一個PFW單位,我們提供的普通股單位的數量將在一對一的基礎上減少。
由於在此次發行中,一份A系列令與普通股單位中包含的每股普通股一起出售,或者是每個PFW單位,因此在此次發行中出售的A系列令數量不會因普通股單位和PFW單位組合的變化而改變。普通股單位中的普通股股份或PFW單位中的預融資憑證(如適用)以及隨附的A系列憑證只能在本次發行中一起購買,但將單獨發行,並在發行後立即分離。
普通股單位和PFW單位都不會被髮行或認證。普通股單位、PFW單位、普通股股份、A系列認購證、預先融資認購證以及由此提供的A系列認購證和預先融資認購證的普通股股份有時在本文中統稱爲「證券」。
我們的普通股在納斯達克上市,代碼爲「AMIX」。2024年10月31日,我們普通股在納斯達克的最後一次報告售價爲每股14.31美元。該基金單位、A系列認購證或預融資認購證尚未建立公開交易市場,我們預計不會發展市場。我們無意在納斯達克或任何其他國家證券交易所或自動報價系統上上市該基金單位、A系列認購證或預融資認購證。如果沒有活躍的交易市場,單位、普通股和預融資股的流動性可能會受到限制。本招股說明書中使用的假設公開發行價格僅用於說明目的。實際發行價可能與招股說明書中使用的假設價格存在重大差異,並將由我們與承銷商之間的談判確定,並且可能不代表實際發行價的價格。
我們是一家「新興成長型公司」,定義見1933年《證券法》(經修訂)第2(a)條(「證券法」),並且我們選擇遵守某些降低的上市公司報告要求。
投資我們的證券涉及高度風險。請參閱標題為的部分 “危險因素” 從本招股說明書第7頁開始,討論與投資我們的證券有關的風險。
美國證券交易委員會或任何其他監管機構均未批准或不批准這些證券,也未傳遞本招股說明書的準確性或充分性。任何相反的陳述都是刑事犯罪。
|
每普通股單位 |
每PFW單位 |
總 |
||||||||||
|
公開發行價 |
$ | $ | $ | |||||||||
|
承保折扣和佣金(1) |
$ | $ | $ | |||||||||
|
收入(不計費用)歸我們所有 (2) |
$ | $ | $ | |||||||||
|
(1) |
代表相當於每個普通股單位或PFW單位8%的承保折扣(如適用)。 |
|
(2) |
我們已同意向承銷商報銷某些費用,並向承銷商代表發出保證書(「代表性配股」)購買本次發行中出售的股份和預融資配股相關普通股股份總數的最多6%,包括超額配股權,但不包括A系列配股權的普通股股份,行使價等於本次發行中出售的普通股單位公開發行價的155%。見「承銷「請參閱第80頁,了解有關承保補償的更多信息。 |
我們已授予承銷商一項選擇權,自本招股說明書之日起最長45天的期限內,以購買最多104,821股我們普通股的額外股份和/或A系列認購權,以承銷商確定的假設公開發行價格購買最多104,822股我們普通股的額外股份或其任何組合,減去承保折扣和佣金,在每種情況下僅用於支付超額分配(如果有的話)。
承銷商預計將於2024年__
拉登堡·塔爾曼
本招股說明書日期爲2024年__
目錄
|
頁面 |
|
|
關於這份招股說明書 |
ii |
|
招股說明書摘要 |
1 |
|
風險因素 |
8 |
|
有關前瞻性陳述的警示說明 |
29 |
|
收益的使用 |
30 |
|
股利政策 |
30 |
|
稀釋 |
31 |
|
大寫 |
32 |
|
管理層對財務狀況和經營成果的討論與分析 |
33 |
|
生意場 |
41 |
|
管理 |
63 |
|
高管和董事薪酬 |
66 |
|
某些關係、關聯人員交易以及董事獨立性 |
73 |
|
某些實益擁有人的擔保所有權以及管理層和有關股東的事項 |
74 |
|
股本說明 |
75 |
|
我們提供的證券說明 |
79 |
|
重要的美國聯邦所得稅考慮因素 |
82 |
|
承銷 |
88 |
|
法律事務 |
93 |
|
專家 |
93 |
|
在那裏您可以找到更多信息 |
94 |
|
財務報表索引 |
96 |
關於這份招股說明書
本招股說明書是我們向美國證券交易委員會(「SEC」)提交的S-1表格註冊聲明的一部分,旨在根據《證券法》登記此處提供的證券。我們還可能提交招股說明書補充書或對註冊聲明的生效後修訂(本招股說明書構成其一部分),其中可能包含與這些發行相關的重要信息。在決定投資我們的證券之前,您應仔細閱讀本招股說明書。
我們沒有、承銷商也沒有授權任何人提供本招股說明書或由我們或代表我們編制的或我們向您推薦的任何自由撰寫招股說明書中所載內容以外的任何信息或任何陳述。我們對其他人可能向您提供的任何其他信息不承擔任何責任,也無法保證其可靠性。本招股說明書僅出售此處提供的證券,並且僅在合法的情況下和司法管轄區內出售。本招股說明書或任何適用的免費撰寫招股說明書中包含的信息僅截至其日期爲最新信息,無論其交付時間或我們證券的任何銷售。自該日期以來,我們的業務、財務狀況、運營業績和前景可能發生了變化。
對於美國以外的投資者:我們和承銷商都沒有采取任何措施允許在任何需要爲此採取行動的司法管轄區(美國除外)進行此次發行或擁有或分發本招股說明書。美國境外持有本招股說明書的人員必須了解並遵守與在美國境外發行證券和分發本招股說明書相關的任何限制。
本招股說明書可能包含對屬於其他實體的商標的引用。僅爲方便起見,本招股說明書中提及的商標和商品名稱,包括徽標、插圖和其他視覺展示,可能沒有®或TM符號。我們無意使用或展示其他公司的商品名稱或商標暗示與任何其他公司存在關係或得到任何其他公司對我們的認可或贊助。
任何交易商、銷售人員或其他人員均無權提供任何信息或代表本招股說明書中未包含的任何內容。您不得依賴任何未經授權的信息或陳述。本招股說明書是一項僅出售在此提供的證券的要約,但僅限於在合法的情況下和在司法管轄區內。本招股說明書中包含的信息僅爲截止日期的最新信息。
本招股說明書包含獨立人士及吾等就市場規模及增長所作的估計及其他統計數據,以及有關本行業的其他數據。本招股說明書中的行業和市場數據來自我們自己的研究以及由第三方進行的行業和一般出版物、調查和研究。這些數據涉及一些假設和限制,幷包含對我們經營的行業未來業績的預測和估計,這些行業受到高度不確定性的影響。我們提醒您不要過分重視此類預測、假設和估計。
招股說明書摘要
本摘要重點介紹了本招股說明書中其他部分包含的信息。此摘要並不包含您在決定投資我們的證券之前應考慮的所有信息。您應該仔細閱讀整個招股說明書,包括“風險因素” 本招股說明書中的部分。本招股說明書中提及 “我們”, “美國”, “它的”, “我們的” 或 “公司” 是Autonomix Medical,Inc.,根據上下文適當。
概述
我們是一家處於開發階段的醫療設備開發公司,專注於推進感知和治療與神經系統相關的疾病的創新技術。我們一流的技術平台包括一個基於導管的微芯片傳感陣列,該陣列可以以高靈敏度檢測和區分神經信號,正如動物研究中所證明的那樣。我們最初正在爲胰腺癌患者開發技術,胰腺癌可能會導致衰弱的疼痛,需要更有效的解決方案。然而,我們相信我們的技術構成了一個平台,有可能解決一系列領域的數十種適應症,包括各種原因的慢性疼痛管理、高血壓、心血管疾病和廣泛的其他神經相關疾病。
我們以最小信號檢測電壓爲單位計算靈敏度,單位爲電極的微伏(uV)時間面積(平方毫米)。這是一個與系統的信號分辨率和空間分辨率有關的組合測量。對於市場上最近的設備BSC Orion來說,信號檢測水平的指標爲10 uV,電極尺寸的指標約爲0.4mm x 0.5mm。對於Autonomix設備,信號檢測水平的指標爲<1uV,電極尺寸的指標約爲0.02mm x 0.03mm。這些指標的差異導致Autonomix設備的靈敏度提高了3,000倍。我們相信,如果我們能夠在臨床試驗中重現這些結果,這將使一種經血管靶向、治療和確認涉及全身神經系統的疾病的治療方法成爲可能,這種方法目前尚不可用,並且可能能夠滿足廣泛的未滿足的醫療需求。
我們的開發工作可以分爲兩個子部分:診斷和治療,其中診斷的重點是感知和識別可能與疾病相關的神經元活動,並具有足夠的精確度,以便通過消融進行靶向治療。我們的傳感導管已經發展到足以在動物模型中證明在消融前成功識別來自特定神經束的信號,並在消融後證實來自經治療的神經的信號終止。我們現在正在改進這種導管的設計,以滿足人類使用所需的標準。在這一努力的同時,我們正在進行首例經血管消融以緩解胰腺癌相關疼痛的人體演示,目的是在未來的關鍵臨床試驗中將傳感和治療結合在一起,使我們的技術能夠商業化啓動。我們是一家處於發展階段的公司,我們還沒有完成任何臨床試驗,不能保證任何試驗的結果都會產生積極的結果,也不能保證結果會支持我們的說法。
我們相信,我們商業化計劃中最要求的方面之一將是從現有的傳感原型擴展到強大的商業版本。如今,我們的傳感設備是手工製造的,包括手工製作和3D打印零件的組合。我們尚未組裝或測試我們擬議設備的商業版本。即使我們提出的設備獲准商業使用,也不能保證我們能夠成功地在商業規模上製造此類設備。
截至2024年6月30日,我們的累計赤字爲4170萬美元,經營活動產生負現金流爲190萬美元,流動資金爲630萬美元,這對我們繼續經營的能力產生了重大懷疑。此外,我們爲實現業務計劃已經並預計將繼續產生巨額成本。我們無法向您保證我們將成功籌集額外資金。除其他外,這些因素對我們繼續作爲持續經營企業的能力提出了極大的懷疑。
最新發展動態
第二季度初步業績(未經審計)
根據目前可用的信息,我們估計截至2024年9月30日,現金及現金等值物約爲520萬美元,截至2024年9月30日第二季度運營中使用的現金爲160萬美元。截至2024年9月30日止六個月的運營使用現金爲340萬美元。
我們對截至2024年9月30日的現金和現金等價物以及2024年第二季度運營中使用的現金的估計是初步的,實際結果可能與這些估計不同,這是因爲我們完成了截至2024年9月30日的三個月和六個月的結算程序,最終調整和從現在到截至2024年9月30日的三個月和六個月的財務結果最終確定之間可能出現的其他發展。因此,這些估計不應被視爲我們根據美國公認會計原則編制的截至2024年9月30日的三個月和六個月的未經審計財務報表的替代品。我們的預期結果可能會發生變化,並不一定表明在截至2024年9月30日的三個月和六個月或任何未來時期將取得的結果。由於上述考慮和本文所述的其他限制,提醒投資者不要過度依賴這一初步財務信息。除非法律要求,否則我們不承擔公開更新或修改這些估計的任何義務。
僱傭協議
2024年6月17日,我們與布拉德·豪澤簽訂了一項僱傭協議,根據協議,豪澤先生同意擔任我們的首席執行官和總裁,最初的三年任期可能會逐年延長。豪瑟先生的協議規定,最初的年度基本工資爲45萬美元(取決於我們薪酬委員會的年度審查和增加),目標年度獎金爲基本工資的60%。根據該協議,豪澤先生被授予一項爲期十年的期權(「激勵期權」),以購買45,000股普通股,行使價相當於我們普通股在僱傭協議簽訂之日的收盤價。該購股權於僱傭協議簽署後四個週年日的每一日分成四個相等的年度分期付款(或每股11,250股股份),前提是豪瑟先生於每個歸屬日期受僱於吾等。如果我們或豪澤先生在沒有「原因」的情況下發生「控制權變更」或終止協議,所有未授予的期權應立即授予。根據納斯達克上市規則第5635(C)(4)條,激勵期權是在我們的2023年股票計劃之外授予的,作爲豪澤先生進入我們的工作的激勵材料。從截至2025年3月31日的年度開始,豪澤先生將有資格獲得由董事會薪酬委員會根據薪酬委員會制定的標準確定的年度期權授予。目標年度期權授予的股票數量將等於1,000,000美元除以授予日我們普通股的每股布萊克-斯科爾斯價值。
2024年6月17日,我們與Lori Bisson簽訂了一項僱傭協議,據此Bisson女士同意擔任我們的執行副主席和首席執行官的戰略顧問(「副主席」),爲期兩年。Bisson女士的協議規定,最初的年度基本工資爲150,000美元(取決於我們薪酬委員會的年度審查和增加),目標年度獎金爲基本工資的50%。根據該協議,Bisson女士繼續按照其先前僱傭協議中所載的歸屬時間表授予擔任首席執行官的Bisson女士和總裁的期權授予。如果發生「控制權變更」或我們或比森女士以「正當理由」無故終止協議的情況,所有未授予的期權應立即授予。比森女士有權獲得截至2024年3月31日的財年的任何激勵性薪酬,包括激勵性薪酬,但截至協議日期尚未支付。從截至2025年3月31日的年度開始,Bisson女士將有資格獲得由董事會薪酬委員會根據薪酬委員會制定的標準確定的年度期權授予。比森女士同意免除與我們於2023年6月30日與她簽訂的先前僱傭協議終止有關的任何應支付給她的遣散費。
許可協議
2024年7月10日,我們與私營醫療技術公司RF Innovation,Inc.(「RFI」)簽訂了一項許可協議(「協議」),授權使用RFI與其Apex 6射頻發生器相關的知識產權的產品(「許可產品」)。Apex 6 Generator是美國食品和藥物管理局(FDA)批准的一項消融技術,旨在損傷神經組織,用於周圍神經系統的疼痛控制。根據該協議,RFI授予我們與許可產品相關的永久非獨家全球免版稅全額支付許可,前提是該許可不包括向客戶銷售某些用於治療脊柱疼痛的產品的權利。關於該協議,我們發行了RFI 12,500股我們普通股的未登記股份作爲許可證的代價。本協議規定,如果我們違反協議中包含的任何陳述、保證或約定,受任何相關補救期限的限制,或者如果我們受到破產或資不抵債事件的影響,RFI有權終止許可證。
未能滿足持續上市規則或標準的通知
於2024年9月16日,我們收到納斯達克證券市場(「納斯達克」)上市資格審核部(「職員」)的短函,通知我們根據「納斯達克上市規則」第5550(A)(2)條(「買入價規則」),本公司普通股的收市價已連續30個交易日收盤低於每股1.00美元的最低收市價。根據納斯達克上市規則第5810(C)(3)(A)條(「合規期規則」),我們已獲提供180個歷日的初步期限,或至2025年3月17日(「合規日」),以恢復遵守投標價格規則。如果在合規日期之前的任何時間,根據合規期規則的要求,我們普通股的收盤價在至少連續10個工作日內收於1.00美元或更高,工作人員將向我們發出書面通知,告知我們遵守投標價格規則,除非工作人員根據納斯達克上市規則第5810(C)(3)(H)條行使其酌情權延長這10天期限。如果我們在2025年3月17日之前沒有遵守投標價格規則,我們可能會獲得第二個180個日曆日的期限來重新獲得遵守。要獲得資格,我們將被要求滿足公開持有的股票市值繼續上市的要求,以及納斯達克資本市場的所有其他初始上市標準,最低投標價格要求除外。此外,我們將被要求通知納斯達克我們打算解決最低投標價格不足的問題,其中可能包括,如有必要,實施反向股票拆分。如果我們未能在合規日期之前重新遵守投標價格規則,並且屆時沒有資格獲得額外的合規期,工作人員將向我們發出書面通知,我們的普通股可能會被除牌。然後,我們將有權就員工的決定向納斯達克上市資格審查委員會提出上訴,並要求舉行聽證會。不能保證,如果我們真的向納斯達克上市資格審查委員會上訴工作人員的退市決定,這種上訴一定會成功。
將事項提交證券持有人投票
2024年10月17日,我們舉行了年度會議,我們的股東批准了我們公司註冊證書的一項修正案(「修正案」),以實現我們普通股流通股的反向股票拆分,拆分比例由我們的董事會在年度會議一週年之前自行決定,在2股1股和1股50股之間。根據我們股東授予的這種授權,我們的董事會批准了我們普通股20股1股的反向股票拆分,並提交了修正案,以實現反向股票拆分。修正案已提交給特拉華州國務卿,根據修正案的條款,反向股票拆分於晚上11點59分生效。東部時間2024年10月24日(「生效時間」)。修正案規定,在生效時,我們每20股已發行和已發行普通股自動合併爲一股已發行和已發行普通股,每股面值不變,仍爲0.001美元。除非上下文另有明確說明,本文中提及的所有股份和每股金額都反映了20股1股的反向股票拆分。
概念驗證人體臨床試驗的初步結果
2024年10月28日,我們在正在進行的概念驗證人體臨床試驗(「試驗」)中強調了前五名「導入」患者的積極初步結果,該試驗評估了輸送經血管能量以消融相關有問題的神經並減輕胰腺癌疼痛患者的疼痛的安全性和有效性。三名患者採用股動脈入路治療,兩名患者採用肱動脈入路治療。所有接受股動脈入路治療的患者對治療反應積極,而接受肱動脈入路治療的患者的疼痛評分沒有改善(或惡化)。使用視覺模擬量表(「視覺模擬量表」)評估,響應者組中的三名患者的疼痛減輕,從手術前的平均評分8.0降至手術後4-6周的平均評分1.33。此外,所有有反應的患者都能夠在手術後4-6周完全停止阿片類藥物的使用。
2024年10月31日,我們重點介紹了前15名患者的陽性初步結果,其中包括五(5)名「引入」患者。股動脈入路11例,臂入路3例。接受股動脈途徑治療的患者對治療反應積極,而接受臂動脈途徑治療的患者疼痛評分沒有改善。一(1)名患者無法接受治療,原因是狹窄程度比篩查前掃描顯示的更嚴重,不包括在修改後的意向治療人群中。應答組11例患者的結果顯示,79%的患者在術後7天VAS疼痛評分(從基線的7.82到2.86)平均疼痛減輕4.96分。通過術後7天的研究,研究顯示阿片類藥物需求減少,沒有有反應的患者需要增加劑量;沒有有反應的患者在術後24小時隨訪後需要阿片類藥物。有反應的患者報告說,在手術後7天,總體健康狀況平均改善了66%。當評估包括無反應者在內的總治療人群時,VAS疼痛評分的平均下降幅度爲3.64分,或48%,從手術前報告的疼痛評分到手術後第7天。
公司信息
我們的主要行政辦公室位於21 Waterway Avenue,Suite 300,The Woodlands,Texas 77380,我們的電話號碼是(713)588-6150。我們的網站地址是www.guardix.com。我們網站上或通過我們網站訪問的信息不屬於本招股說明書的一部分。
風險因素摘要
我們的業務面臨多種風險,您在做出投資決定之前應該了解這些風險。您應仔細考慮本招股說明書中列出的所有信息,特別是在決定是否投資我們的普通股之前,應評估標題爲「風險因素」的部分中列出的具體因素。這些重要風險包括以下:
與我們整體業務相關的風險
|
● |
我們的獨立註冊會計師事務所的報告對我們繼續作爲持續經營企業的能力表示嚴重懷疑。 |
|
● |
我們沒有批准的產品,我們無法向您保證我們未來將產生收入或盈利。 |
|
● |
我們打算利用單一製造商來製造我們的主要候選產品,並希望繼續這樣做商業產品。與我們產品製造相關的風險可能會降低我們的毛利率並對我們的經營業績產生負面影響。 |
|
● |
我們是一家發展階段的公司,尚未有創收的歷史。 |
|
● |
我們的業務可能會受到全球經濟狀況、全球金融市場的不確定性以及可能的貿易關稅和貿易限制的不利影響。 |
|
● |
我們沒有組裝和測試產品的經驗,產品組裝過程中可能會遇到問題或延遲,或者無法滿足某些監管要求,這可能會對我們的業務和財務業績造成不利影響。 |
|
● |
生命科學領域快速變化的技術可能會使我們正在開發的產品過時。 |
|
● |
影響金融服務業的不利事態發展,例如涉及流動性、違約或金融機構或交易對手方不履行義務的實際事件或擔憂,可能會對我們當前和預計的業務運營以及我們的財務狀況和運營結果產生不利影響。 |
|
● |
災難性事件和災難恢復可能會中斷業務連續性。 |
|
● |
我們可能無法滿足薩班斯-奧克斯利法案的規定,並且可能缺乏上市公司所需的財務控制和保障措施。 |
|
● |
雖然我們公司的管理層正在努力改善我們的內部控制和程序,但目前管理層已確定我們的內部控制被認爲不充分,這可能會導致我們的財務報告不可靠並導致錯誤信息被傳播給公衆。 |
與政府監管和產品批准相關的風險
|
● |
無法保證FDA將授予我們未來產品的510(k)或重新許可或上市前批准申請(「PMA」),並且未能爲我們未來產品獲得必要的許可或批准將對我們發展業務的能力產生不利影響。 |
|
● |
對我們未來產品的修改可能需要新的監管許可或批准,或者可能要求我們召回或停止營銷我們的產品,直到獲得許可或批准。 |
|
● |
我們未來臨床試驗的結果可能不支持我們的候選產品主張,或者可能導致發現不良副作用。 |
|
● |
我們計劃在美國境外進行初步概念驗證試驗,並將該試驗的相關數據提交給FDA,以儘量減少美國批准的臨床要求。無法保證FDA會接受這些數據。 |
|
● |
我們的臨床研究可能會被推遲或受到許多因素的不利影響。 |
|
● |
即使我們的產品已獲得FDA批准或批准,如果我們或我們的供應商未能遵守FDA的持續要求,或者如果我們的產品遇到意外問題,這些產品也可能會受到限制或退出市場。 |
|
● |
我們的產品未來可能會被產品召回,這可能會損害我們的業務和財務業績。 |
|
● |
如果我們的產品導致或導致死亡或重傷,或以某些方式發生故障,我們將遵守醫療器械報告法規,這可能會導致自願糾正行動或強制執行行動。 |
|
● |
用於製造我們設備的某些零部件可能會出現全球供應短缺的情況,這可能會影響我們爲客戶製造設備或維持研發時間表的能力。 |
|
● |
美國立法或FDA監管改革可能會使我們獲得候選產品的監管批准以及在批准後製造、營銷和分銷我們的產品變得更加困難和成本。 |
|
● |
由於我們業務的專業性,終止與我們的關鍵員工、顧問和顧問的關係可能會阻止我們成功運營我們的業務,包括開發我們的產品、進行臨床研究、將我們的產品商業化和獲得任何必要的融資。 |
|
● |
未能從第三方付款人獲得和維持足夠的保險範圍和報銷可能會對我們產品(如果獲得批准)的接受產生不利影響,並減少我們的收入。 |
|
● |
我們可能無法成功確保和維護必要的報銷代碼,以促進準確、及時地爲我們的產品或與我們產品相關的醫生服務計費。 |
|
● |
如果我們無法與醫生建立良好的關係,我們的業務可能會受到負面影響。 |
|
● |
無法保證Medicare或Medicare行政承包商將爲我們的產品提供保險或足夠的付款率。 |
與我們的知識產權和信息技術相關的風險
|
● |
如果第三方計算機系統故障、第三方系統遭受網絡攻擊或網絡安全缺陷,我們的業務和運營將受到影響。 |
|
● |
人工智能帶來了可能影響我們業務的風險和挑戰,包括對我們的機密信息、專有信息和個人數據構成安全風險。 |
|
● |
網絡安全風險和網絡事件可能會對我們的業務產生不利影響並擾亂運營。 |
|
● |
我們可能會因與專利和其他知識產權相關的訴訟而產生巨額費用。 |
|
● |
如果第三方聲稱我們的產品侵犯了他們的知識產權,我們可能被迫花費大量財務資源和管理時間來抵禦此類行爲,我們的財務狀況和運營業績可能會受到影響。 |
|
● |
如果我們無法保護我們產品中使用的知識產權,其他人可能會複製我們的創新,這可能會損害我們在市場中有效競爭的能力。 |
|
● |
我們可能會受到指控,稱我們的員工錯誤地使用或披露了其前僱主據稱的商業機密。 |
|
● |
如果我們不能保護我們的專有信息和專有技術的機密性,我們的技術和產品的價值可能會受到不利影響。 |
與此次發行相關的風險
| ● | 對截至2024年9月30日期間的業績進行初步估計存在重大限制。我們的獨立註冊會計師事務所尚未對初步未經審計的結果進行審計或審查,也不對初步未經審計的結果發表意見或任何其他形式的保證。我們或我們的獨立註冊會計師事務所可能會發現需要我們對持續經營業務的營業虧損和所得稅前淨虧損的初步估計進行調整的項目。 |
|
● |
我們在如何使用此次發行的收益方面擁有廣泛的自由裁量權,可能無法有效地使用這些收益,這可能會影響我們的運營結果,並導致我們的普通股下跌。 |
|
● |
我們將需要大量資金,但我們可能無法以可接受的條件獲得這些資金,或者根本無法獲得這些資金,如果無法獲得這些資金,可能會要求我們推遲、限制、減少或停止我們的運營。 |
|
● |
此次發行的買家將立即經歷淨有形淨資產的大幅稀釋。 |
|
● |
如果增發股本以籌集資本、爲收購融資或與戰略交易相關,您的所有權可能會被稀釋。 |
|
● |
如果我們的股價在上市後出現波動,您的投資可能會損失很大一部分。 |
|
● |
本次發行可能會導致我們普通股的交易價格下跌。 |
|
● |
我們從未爲我們的股本支付過股息,我們預計在可預見的未來也不會支付股息。 |
與我們普通股相關的風險
| ● | 我們於2024年10月24日完成了反向股票分拆,以重新遵守納斯達克上市規則,我們無法預測這種反向股票分拆將對我們普通股股票的市場價格產生的影響。 |
|
● |
我們普通股的所有權集中在我們現有的高管和董事手中可能會阻止新投資者影響重大公司決策。 |
|
● |
賣空者使用的技術未來可能會壓低我們普通股的市場價格。 |
|
● |
如果證券或行業分析師不發佈有關我們的研究或報告,或者如果他們對我們普通股的建議做出不利改變,那麼我們的股價和交易量可能會下降。 |
|
● |
作爲一家根據JumpStart Our Business Startups Act或JOBS Act的「新興成長型公司」,我們被允許並打算依賴於某些披露要求的豁免。 |
|
● |
我們未能滿足納斯達克的持續上市要求可能會導致我們的普通股退市。 |
|
● |
我們的註冊證書包括論壇選擇條款,這可能會導致原告在針對我們的任何訴訟中獲得不利的結果。 |
|
● |
作爲一家上市公司的要求可能會使我們的資源緊張,分散管理層的注意力,並影響我們吸引和留住合格董事會成員的能力。 |
|
● |
我們可能面臨更大的證券集體訴訟風險。 |
供品
|
我們提供的公共單元 |
最多698,812個普通股單位,每個普通股單位由一股我們的普通股和一份普通權證組成,假設公開發行價爲每股普通股單位14.31美元,這等於我們的普通股在2024年10月31日公佈的最後銷售價格。
普通股單位最初不會以獨立形式進行認證或發行。普通股股份和組成普通股單位的普通權證在發行時立即可以分離,並將在本次發行中單獨發行。 |
|
緊接本次發行前已發行的普通股 |
1,152,149股 |
|
本次發行後緊接發行的普通股 |
1,850,961股普通股(如果承銷商行使其超額配售選擇權,則爲1,955,783股),不包括在行使普通權證時可發行的普通股,並假設沒有出售任何預融資權證。 |
|
提供全氟化水機組 |
我們也向那些在本次發售中購買普通股單位的購買者(如果有)提出要約,否則購買者連同其聯屬公司和某些關聯方將在本次發售完成後立即實益擁有超過4.99%(或在購買者選擇時,9.99%)我們的已發行普通股,如果該等購買者選擇的話,有機會購買最多_個普通股單位,以代替普通股單位,否則將導致任何該等購買者的實益所有權超過我們的已發行普通股的4.99%。每個PFW單位包括:(I)購買一股我們普通股的預籌資金認股權證和(Ii)一份A系列認股權證。每個PFW單位的收購價將等於在這次發行中向公衆出售普通股單位的公開發行價減去0.001美元,每個預先出資的認股權證的行使價將爲每股0.001美元。見「發行證券說明」。本招股說明書還涉及在行使預融資權證時可發行的股票的發行。
對於我們出售的每一個PFW單位,我們提供的普通股單位的數量將在一對一的基礎上減少。由於在本次發售中,A系列認股權證將與每個普通股單位和每個PFW單位一起出售,因此,本次發售中出售的A系列認股權證的數量不會因普通股單位和PFW單位的銷售組合發生變化而發生變化。本次發行還涉及在行使預融資認股權證時發行普通股。 |
| 首輪認股權證 |
在本次發行中購買的每個普通股單位或PFW單位,視情況而定,將包括一個A系列認股權證,以購買我們普通股的一股。A系列認股權證的行使價爲每股普通股_普通股單位的普通股股份或PFW單位的預籌資權證(視情況而定),以及隨附的A系列認股權證,只能在本次發行中一起購買,但將單獨發行,並將在發行時立即分開。本次發行還涉及在行使A系列認股權證時可發行的普通股的發行。每份A系列認股權證適用於一股普通股,在發生影響我們普通股的股息、股票拆分、股票合併、重新分類、重組或類似事件時,可進行調整。
A系列認股權證持有人不得行使A系列認股權證的任何部分,條件是持有人連同其聯屬公司及作爲一個集團行事的任何其他個人或實體,在行使後將持有超過4.99%(或經持有人選擇,該限額可增加至最高9.99%)的已發行普通股,因爲該所有權百分比是根據A系列認股權證的條款厘定的,但在持有人向吾等發出通知後,持有人可免除不超過9.99%的限制。本招股說明書還涉及在行使首輪認股權證後可發行的普通股的發售。
爲了更好地理解普通權證的條款,您應該仔細閱讀本招股說明書的「我們提供的證券說明」部分。你還應該閱讀普通權證的表格,它是作爲包括本招股說明書在內的註冊聲明的證物提交的。 |
|
超額配售選擇權 |
我們已向承銷商授予爲期45天的選擇權,自本招股說明書公佈之日起,以相當於每股公開發行價減去承銷折扣的每股額外股份收購價,購買最多104,822股普通股和/或104,822股普通權證或其任何組合。
由於普通權證不會在國家證券交易所或其他國家認可的交易市場上市,承銷商在不行使承銷商關於普通權證的超額配售選擇權的情況下,將無法滿足任何超額配售股票和普通權證的要求。因此,承銷商將對首次發售股份和普通權證時超額配售的所有普通權證行使超額配售選擇權。然而,由於我們的普通股是公開交易的,承銷商可以通過在公開市場購買股票來滿足我們普通股的部分或全部超額配售,而不會有義務對我們的普通股行使超額配售選擇權。 |
|
禁售協議 |
我們已與承銷商達成協議,在本次發行生效日期後的90天內不再出售額外的股權證券。我們的董事和高級管理人員已與承銷商達成協議,在本招股說明書日期後90天內,不得出售、出售、簽訂出售合同、質押或以其他方式處置其持有的任何普通股或可轉換爲我們普通股的證券,承銷商可酌情免除這一限制。 |
|
代表權證 |
我們已同意向承銷商代表發行認股權證,以購買本次發行中出售的標的單位總數的6%,包括與PFW單位相關的股份,或41,928股普通股(或48,218股普通股,假設超額配售選擇權全部行使),作爲與此次發行相關而應支付給承銷商的補償的一部分。代表認股權證將可於任何時間及不時全部或部分行使,行使價相等於本次發售所售股份公開發行價的155%,於本次發售開始發售後180天開始全部或部分行使,於本次發售開始發售五週年時屆滿,在其他方面與發售中發行的普通權證實質上相似。代表認股權證和代表認股權證相關的普通股股份正在註冊說明書上登記,招股說明書是其中的一部分。見本招股說明書第80頁的「承銷」部分。 |
|
收益的使用 |
我們估計此次發行的淨收益將約爲900萬美元,這是根據假設的每股14.31美元的公開發行價格(即2024年10月31日納斯達克報告的我們普通股的收盤價)計算的,扣除承保折扣和我們應付的估計發行費用。我們打算將此次發行的收益主要用於資助我們的臨床試驗、其他研究和開發、知識產權開發和運營資金。請參閱「收益的使用」。 |
|
風險因素 |
投資我們的證券涉及高度風險。請參閱本招股說明書第7頁開始的「風險因素」以及本招股說明書中通過引用包含的其他信息,以討論您在決定投資我們的證券之前應仔細考慮的風險因素。 |
|
納斯達克上市標誌 |
我們的普通股在納斯達克資本市場上市,代碼爲「AMIX」。 |
此後將發行的普通股股數基於截至2024年10月1日發行的1,152,120股,不包括:
|
● |
112,534股普通股未償憑證,加權平均行使價爲每股8.26美元; |
|
● |
216,483股普通股未行使期權,加權平均行使價爲每股36.43美元; |
|
● |
33,250股普通股未發行可轉換票據,加權平均換股價爲每股40.00美元; |
|
● |
Autonomix Medical,Inc.未來可發行75,633股股票2023年股票計劃; |
|
● |
698,812股本次發行中可發行的普通股普通股;以及 |
|
● |
與本次發行相關的代表性令狀可向承銷商發行41,928股普通股。 |
除另有說明外,本招股說明書中的信息假設不會行使期權或認購權,也不會轉換可轉換票據。
除非另有說明,本招股說明書反映並假定承銷商不行使其超額配售選擇權。
上面討論的信息僅爲說明性信息,並將根據實際公開發行價格和定價時確定的本次發行的其他條款進行調整。
風險因素
投資我們的證券涉及高度風險。在投資我們的證券之前,您應仔細考慮下文討論的風險和不確定性 “風險因素”.在決定購買我們的證券之前,您應仔細考慮以下每項風險,以及本招股說明書中列出的所有其他信息,包括財務報表和相關注釋。如果實際發生以下任何一種風險,我們的業務可能會受到損害。在這種情況下,我們普通股的交易價格可能會下降,您可能會損失全部或部分投資。
與我們整體業務相關的風險
我們的獨立註冊會計師事務所日期爲2024年5月31日的報告對我們繼續作爲持續經營企業的能力表示嚴重懷疑。
截至2024年6月30日,我們累計赤字爲4170萬美元,經營活動產生的負現金流爲190萬美元,流動資金爲630萬美元。我們的獨立註冊會計師事務所日期爲2024年5月31日的報告對我們繼續作爲持續經營企業的能力表示嚴重懷疑。此外,我們在實現業務計劃時已經並預計將繼續產生大量成本。我們無法向您保證我們籌集足夠資金爲業務提供資金的計劃將會成功。除其他外,這些因素對我們繼續作爲持續經營企業的能力提出了極大的懷疑。本招股說明書其他地方包含的財務報表不包括因我們無法籌集額外資本或無法繼續持續經營而可能導致的任何調整。
我們沒有批准的產品,我們無法向您保證我們未來將產生收入或盈利。
我們的產品可能永遠不會獲得美國食品藥品監督管理局(「FDA」)或任何其他外國監管機構的批准,也不會成爲商業可行或被接受使用。自成立以來,我們已經遭受了重大損失,預計在可預見的未來將繼續經歷運營損失和負現金流。我們預計將投入大量資源用於招聘人員、持續的科學和產品研究與開發、產品測試以及臨床前和臨床研究、知識產權開發和起訴、營銷和推廣、資本支出、流動資金、一般和行政費用以及與我們的資本籌集工作相關的費用和費用。我們預計將產生與諮詢費用、僱用科學家、工程師、科學和其他運營人員以及持續發展與戰略合作伙伴關係相關的成本和費用。
我們打算利用單一製造商來製造我們的主要候選產品,並希望繼續這樣做商業產品。與我們產品製造相關的風險可能會降低我們的毛利率並對我們的經營業績產生負面影響。
我們沒有任何製造設施或直接製造人員。我們目前依賴並預計將繼續依賴單一製造商來生產我們的商業製造主要候選產品。因此,我們面臨與依賴單一製造商相關的衆多風險。如果他們在製造我們的候選產品時遇到問題,那麼我們的業務可能會受到重大影響。這些問題包括:
|
● |
無法及時確保產品組件、數量不足或商業上合理的條款; |
|
● |
未能增加產量以滿足需求; |
|
● |
無法修改生產線以使我們能夠有效地生產未來的產品或根據監管要求對當前產品實施更改; |
|
● |
難以及時識別和認證替代製造商; |
|
● |
無法與未來的第三方製造商達成協議或無法以可接受的條款達成協議;或 |
|
● |
可能對我們製造商的設備或設施造成損壞或破壞。 |
如果對我們未來產品的需求增加,我們的製造商將需要投資額外資源來購買零部件、僱用和培訓員工並增強他們的製造流程。如果他們未能有效提高產能,我們的銷售額可能無法按照預期增長,我們的營業利潤率可能會波動或下降。我們沒有與製造商達成長期協議,也不能保證他們未來會繼續爲我們提供製造服務。
我們是一家發展階段的公司,尚未有創收的歷史。
作爲一家處於發展階段的實體,我們尚未產生任何收入。投資者要承受新業務創建和發展所帶來的所有風險,每個投資者都應該準備好承受投資的完全損失。我們尚未走出開發階段,可能無法進一步籌集股權。這些因素讓人們對我們繼續經營的能力產生了極大的懷疑。
我們的業務可能會受到全球經濟狀況、全球金融市場的不確定性以及可能的貿易關稅和貿易限制的不利影響。
我們的業務和業績將在很大程度上取決於世界經濟和地緣政治條件。全球經濟狀況的不確定性可能會導致潛在客戶推遲購買我們未來的產品,以應對信貸緊縮、失業、負面金融消息和/或收入或資產價值下降和其他宏觀經濟因素,這可能對我們未來產品的需求產生重大負面影響,從而對我們的業務、運營業績或財務狀況產生重大負面影響。例如,當前全球金融市場繼續反映不確定性,新冠肺炎疫情、俄羅斯和烏克蘭之間持續的軍事衝突以及以色列持續的衝突加劇了這種不確定性。鑑於這些不確定性,全球經濟、金融市場和消費者信心可能會受到進一步破壞。如果經濟狀況意外惡化,我們的業務和經營業績可能會受到實質性的不利影響。例如,我們未來的客戶,包括我們的分銷商及其客戶,可能難以獲得支持歷史或預計採購模式所需的營運資金和其他融資,這可能會對我們的運營結果產生負面影響。
最近的全球經濟放緩可能會持續下去,並可能導致某些經濟體陷入經濟衰退,包括美國。此外,包括美國在內的世界各地通貨膨脹上升給我們的成本帶來了壓力。經濟持續放緩或衰退以及通脹壓力可能會對我們的業務產生負面影響,包括需求減少、成本增加和其他挑戰。政府爲解決經濟放緩和通脹上升而採取的行動,包括提高利率,也可能對我們的增長造成負面影響。
美國和中國之間的總體貿易緊張局勢一直在升級,美國對中國等潛在國家的商品徵收關稅造成的任何經濟和政治不確定性,以及中國或其他國家作爲回應的任何相應關稅或貨幣貶值,都可能對我們未來的產品產生負面影響、需求和/或增加成本。此外,俄羅斯在2022年初入侵烏克蘭引發了美國和歐洲國家的重大制裁。由此導致的美國貿易政策變化可能引發俄羅斯、其盟友和包括中國在內的其他受影響國家採取報復行動,導致潛在的貿易戰。此外,如果俄羅斯和烏克蘭之間的衝突持續很長一段時間,或者如果包括美國在內的其他國家捲入衝突,我們可能會面臨對我們的業務和財務狀況的重大不利影響。例如,如果我們的供應或客戶安排因擴大制裁或未來我們有業務或關係的國家的參與而中斷,我們的業務可能會受到實質性干擾。此外,作爲衝突的一部分,網絡攻擊的使用可能會擴大,這可能會對我們維持或加強網絡安全和數據保護措施的能力產生不利影響。
未來無法從債務或資本來源獲得足夠的融資可能會迫使我們自籌資金戰略計劃,甚至放棄某些機會,這反過來又可能會損害我們的業績。
我們沒有組裝和測試產品的經驗,產品組裝過程中可能會遇到問題或延遲,或者無法滿足某些監管要求,這可能會對我們的業務和財務業績造成不利影響。
我們沒有組裝和測試計劃中的設備的經驗,也沒有商業規模這樣做的經驗。爲了實現盈利,我們必須按照監管要求並以可接受的成本以商業批量組裝和測試我們計劃的設備。提高我們在商業規模上組裝和測試產品的能力將需要我們提高內部效率。我們在提高組裝和測試能力時可能會遇到一些困難,包括:
|
● |
管理生產產量; |
|
● |
保持質量控制和保證; |
|
● |
提供組件和服務可用性; |
|
● |
維持適當的控制政策和程序; |
|
● |
僱用和留住合格的人員;以及 |
|
● |
遵守州、聯邦和外國法規。 |
如果由於我們無法組裝和測試計劃設備而無法滿足對計劃設備的商業需求,我們產生收入的能力將會受到損害,市場對我們產品的接受度可能會受到不利影響,客戶可能會購買或使用我們競爭對手的產品。
生命科學領域快速變化的技術可能會使我們正在開發的產品過時。
總體而言,醫療器械和生命科學行業的特點是快速而重大的技術變革、頻繁的新產品推出和改進以及不斷髮展的行業標準。我們未來的成功將取決於我們不斷開發並改進我們設計的產品的能力,以及開發和推出新產品以及時且具有成本效益的方式滿足客戶不斷變化的需求的能力。
影響金融服務業的不利事態發展,例如涉及流動性、違約或 不履行 金融機構或交易對手方的行爲可能會對我們當前和預計的業務運營以及我們的財務狀況和運營業績產生不利影響。
實際事件涉及流動性有限、違約、不履行、風險或其他不利發展,影響金融機構、交易對手或金融服務業或金融服務業的其他公司,或對任何此類事件或其他類似風險的擔憂或傳言,過去曾發生,未來可能導致整個市場的流動性問題。例如,2023年3月10日,硅谷銀行(SVB)被加州金融保護和創新部關閉,後者任命聯邦存款保險公司(FDIC)爲接管人。同樣,2023年3月12日,簽名銀行(Signature Bank Corp.)和銀門資本(Silvergate Capital Corp.)都被捲入破產管理程序。儘管財政部、聯儲局和聯邦存款保險公司已確保SVB的所有儲戶在關閉僅一個工作日後就可以提取他們的所有資金,包括無保險存款帳戶中持有的資金、信貸協議下的借款人、信用證和某些其他金融工具,但被聯邦存款保險公司接管的SVB、Signature或任何其他金融機構可能無法提取其下的未提取金額。儘管吾等並非任何重大信用證或任何其他具SVB、Signature或任何其他金融機構目前處於接管狀態的金融機構的借款人或一方,但如果吾等訂立任何該等票據,而該等票據的任何貸款人或交易對手將被接管,吾等可能無法取得該等資金。此外,如果我們的任何合作伙伴、供應商或與我們有業務往來的其他方無法根據此類工具或與此類金融機構的貸款安排獲得資金,這些各方向我們支付債務或達成要求向我們支付額外款項的新商業安排的能力可能會受到不利影響。在這方面,與這些金融機構簽訂的信貸協議和安排的對手方,以及諸如信用證受益人等第三方,可能會受到這些金融機構關閉的直接影響,更廣泛的金融服務業對流動性的擔憂仍然存在不確定性。類似的影響過去也曾發生過,例如在2008-2010年金融危機期間。通貨膨脹和利率的快速上升導致之前發行的利率低於當前市場利率的政府債券的交易價值下降。儘管美國財政部、聯邦存款保險公司和聯邦儲備委員會已經宣佈了一項計劃,向以金融機構持有的某些此類政府證券爲擔保的金融機構提供高達250億美元的億貸款,以降低出售此類工具可能造成的潛在損失的風險,但金融機構對客戶提款的廣泛需求或金融機構對立即流動性的其他流動性需求可能會超出該計劃的能力。
我們獲得的資金來源和其他信貸安排的金額足以爲我們目前和預計的未來業務運營提供資金或資本化,這可能會受到影響我們、我們直接與之簽訂信貸協議或安排的任何金融機構、或整個金融服務業或整體經濟的因素的嚴重影響。這些因素可能包括,除其他外,諸如流動性限制或失敗、根據各種金融、信貸或流動性協議或安排履行義務的能力、金融服務業或金融市場的中斷或不穩定,或對金融服務業公司前景的擔憂或負面預期。這些因素可能涉及與我們有金融或業務關係的金融機構或金融服務業公司,但也可能包括涉及金融市場或金融服務業的一般因素。
涉及其中一個或多個因素的事件或擔憂的結果可能包括對我們當前和預計的業務運營以及我們的財務狀況和運營業績的各種重大和不利影響。這些風險包括但不限於以下:
|
● |
延遲獲得存款或其他金融資產或未投保的存款或其他金融資產的損失; |
|
● |
無法獲得信貸安排或其他流動資金資源; |
|
● |
潛在或實際違反合同義務,要求我們維持信用證或其他信用支持安排;或 |
|
● |
終止現金管理安排和/或延遲獲得或實際損失受現金管理安排制約的資金。 |
此外,投資者對美國或國際金融體系的擔憂可能會導致不太有利的商業融資條款,包括更高的利率或成本以及更嚴格的財務和運營契約,或者對獲得信貸和流動性來源的系統性限制,從而使我們更難以可接受的條款獲得融資,甚至根本不融資。除其他風險外,任何可用資金或現金和流動性資源的減少都可能對我們履行運營費用或其他義務(財務或其他方面)的能力造成不利影響,導致違反我們的財務和/或合同義務,或導致違反聯邦或州工資和工時法。上述任何影響,或由上述因素或其他相關或類似因素所導致的任何其他影響,均可能對我們的流動資金及我們當前及/或預期的業務營運、財務狀況及營運業績產生重大不利影響。
此外,宏觀經濟或金融服務業的任何進一步惡化都可能導致我們的合作伙伴、供應商或供應商的損失或違約,進而可能對我們當前和/或預期的業務運營以及運營結果和財務狀況產生重大不利影響。例如,合作伙伴可能在到期時無法付款、根據與我們的協議違約、破產或宣佈破產,或者供應商可能決定不再作爲客戶與我們打交道。此外,供應商或供應商可能會受到上述任何流動性或其他風險的不利影響,這些風險可能會對我們造成重大不利影響,包括但不限於延遲獲得或失去獲得未投保存款的機會,或失去利用涉及陷入困境或破產的金融機構的現有信貸安排的能力。任何合作伙伴、供應商或供應商的破產或資不抵債,或任何合作伙伴未能在到期時付款,或合作伙伴、供應商或供應商的任何違約或違約,或任何重大供應商關係的喪失,都可能導致我們遭受重大損失,並可能對我們的業務產生重大不利影響。
災難性事件和災難恢復可能會中斷業務連續性。
如果發生自然災害或惡劣天氣事件,包括但不限於地震、野火、乾旱、洪水、龍捲風、颶風或海嘯、健康流行病(例如我們的員工隊伍中爆發流感)或人爲災難性事件,我們的系統或運營中斷或故障,可能會導致完成銷售、持續生產或履行我們業務的其他關鍵職能的延遲,特別是如果我們的場所發生災難性事件。全球氣候變化可能導致某些自然災害發生得更頻繁或強度更大。任何此類事件都可能嚴重影響我們開展正常業務運營的能力,因此我們的經營業績可能會受到不利影響。還可能存在不可預見的次要影響,例如對我們客戶的影響,這可能會導致新訂單延遲、完成銷售延遲,甚至訂單取消。
我們可能無法滿足薩班斯-奧克斯利法案的規定,並且可能缺乏上市公司所需的財務控制和保障措施。
確保我們有足夠的內部財務和會計控制和程序,以便及時編制準確的財務報表,這是一項既昂貴又耗時的工作,需要經常重新評估。我們的管理層得出結論認爲,我們對財務報告的內部控制是無效的,並發現我們的內部控制在缺乏職責分工、一般技術控制和財務報表報告等方面存在重大弱點。雖然管理層正在努力補救重大弱點,但不能保證在經濟上可行和可持續的情況下,這種變化將補救已確定的重大弱點,也不能保證控制措施將防止或發現未來的重大弱點。如果我們不能對財務報告保持有效的內部控制,我們的財務報表,包括相關的披露,可能會不準確,這可能會對我們的業務產生實質性的不利影響。我們可能會發現我們的內部財務和會計控制和程序存在更多重大弱點,需要不時加以改進。
管理層負責建立和維持對財務報告的充分內部控制,以合理保證我們財務報告的可靠性以及根據美國公認會計原則爲外部目的編制財務報表。管理層預計我們對財務報告的內部控制不會防止或檢測所有錯誤和所有欺詐。無論設計和操作得多麼好,控制系統只能提供合理而非絕對的保證來滿足控制系統的目標。由於所有控制系統固有的侷限性,對控制措施的評估無法絕對保證不會發生因錯誤或欺詐而導致的錯誤陳述,或者我們公司內部的所有控制問題和欺詐實例(如果有的話)都將被檢測到。
從截至2025年3月31日的年度10-k表格和10-Q表格開始,我們必須遵守《薩班斯-奧克斯利法案》第404條的規定。我們預計將花費大量資源來制定第404條要求的必要文檔和測試程序。我們無法確定我們將採取的行動來改善財務報告的內部控制是否足夠,或者我們是否能夠及時實施我們計劃的流程和程序。此外,如果我們無法及時編制準確的財務報表,投資者可能會對我們財務報表的可靠性失去信心,這可能會導致我們普通股的市場價格下跌,並使我們更難爲我們的運營和增長提供資金。
而我們的公司’管理層正在努力改善我們的內部控制和程序,目前管理層已確定我們的內部控制被認爲不充分,這可能導致我們的財務報告不可靠並導致錯誤信息傳播給公衆。
我們的管理層負責建立和維護對財務報告的充分內部控制。根據修訂的1934年證券交易法第13 a-15(f)條的定義(「交易法」),財務報告內部控制是由首席執行官和首席財務官設計或監督的過程,並由董事會、管理層和其他人員實施,根據公認的會計原則,爲財務報告的可靠性和爲外部目的編制財務報表提供合理保證,幷包括以下政策和程序:
|
● |
與保存合理、詳細、準確和公平地反映我們資產的交易和處置的記錄有關; |
|
● |
合理保證交易被記錄爲根據公認會計原則編制財務報表所需,並且公司的收入和支出僅根據公司管理層和/或董事的授權進行;以及 |
|
● |
就防止或及時發現可能對財務報表產生重大影響的未經授權收購、使用或處置公司資產提供合理保證。 |
我們需要包括一份關於我們對財務報告內部控制有效性的管理報告。爲了遵守管理認證要求而執行所需的系統和流程評估、測試和補救,我們預計會產生額外的費用並轉移管理層的時間。
目前,我們沒有足夠數量的員工來區分責任,並且可能無力增加員工或聘請外部顧問或專業人士來解決我們缺乏員工的問題。在測試過程中,我們可能會發現我們可能無法及時補救的其他缺陷。如果我們無法提供可靠的財務報告或防止欺詐,我們的業務和經營成果可能會受到損害,投資者可能會對我們報告的財務信息失去信心,如果市場發展,我們普通股的交易價格可能會大幅下跌。
我們的公司註冊證書和章程(迄今爲止修訂的每一項)規定了對高級職員和董事的賠償,費用由公司承擔,並限制了他們可能給我們帶來重大成本並損害我們股東利益的責任,因爲公司資源可能會被用來爲高級職員和/或董事的利益。
我們的公司註冊證書和章程(迄今爲止修訂的)均規定了對我們的高級職員和董事的賠償。我們獲悉,美國證券交易委員會認爲,對聯邦證券法下產生的責任的賠償違反了《證券法》中規定的公共政策,因此不可執行。
與政府監管和產品批准相關的風險
無法保證FDA將授予510(k)或重新獲得許可或上市前批准申請(“PMA”)我們未來的產品以及未能爲我們未來的產品獲得必要的許可或批准將對我們發展業務的能力產生不利影響。
我們的主要候選產品將需要FDA批准510(k)或重新分類申請,或者可能需要FDA批准PMA。FDA可能不會批准或批准這些產品用於成功商業化所需或理想的適應症。事實上,FDA可能會拒絕我們對新產品、新預期用途或現有產品修改的上市前許可或上市前批准的請求。未能獲得對我們產品的許可或批准將對我們繼續或擴大業務的能力產生不利影響。
如果我們未能獲得並維持監管機構的批准和許可,或者無法獲得FDA對我們的設備、我們未來的產品或產品改進的批准或批准,或者在獲得FDA的批准或批准方面經歷重大延遲,我們商業分銷和營銷這些產品的能力可能會受到影響。
我們的產品將受到FDA和許多其他聯邦、州和外國政府機構的嚴格監管。獲得監管部門的批准或批准將醫療設備推向市場的過程可能既昂貴又耗時,而且我們可能無法及時獲得這些批准或批准,如果根本沒有的話。特別是,FDA僅允許在新醫療設備獲得聯邦食品、藥物和化妝品法案第510(K)條規定的許可後,或在獲得批准的上市前批准申請(PMA)後,才允許該設備進行商業分銷,除非該設備特別豁免這些要求。如果製造商證明新產品與其他通過510(K)認證的產品基本相同,FDA將通過510(K)流程批准低風險醫療器械的營銷,如果沒有實質性同等產品,則通過從頭開始流程。被認爲構成最大風險的高風險設備,如維持生命的、維持生命的或可植入的設備,或不被認爲與先前清除的設備基本等同的設備,需要獲得PMA的批准。與510(K)或從頭放行程序相比,PMA程序更昂貴、更漫長、更不確定。PMA的應用必須有廣泛的數據支持,包括但不限於技術、臨床前、臨床試驗、製造和標籤數據,以向FDA滿意地證明該設備用於其預期用途的安全性和有效性。我們相信,我們目前的候選產品將需要通過510(K)或從頭開始流程的批准。
對我們未來產品的修改可能需要新的監管許可或批准,或者可能要求我們召回或停止營銷我們的產品,直到獲得許可或批准。
對我們未來產品的修改可能需要新的監管批准或許可,包括510(K)許可或上市前批准,或者要求我們召回或停止銷售修改後的設備,直到獲得這些許可或批准。FDA要求設備製造商最初確定一項修改是否需要新的批准、補充或批准,並將其記錄在案。製造商可以確定,修改不會顯著影響安全性或有效性,也不代表其預期用途發生重大變化,因此不需要新的510(K)許可。然而,FDA可以審查製造商的決定,並可能不同意。FDA也可以主動決定需要新的批准或批准。一旦我們有了商業化的產品,我們可能會在未來進行我們認爲不需要或不需要額外批准或批准的修改。如果FDA不同意並要求對這些修改進行新的許可或批准,我們可能會被要求召回並停止以修改的形式銷售我們的產品,這可能要求我們重新設計產品並損害我們的運營結果。在這種情況下,我們可能會受到重大執法行動的影響。
如果我們確定對產品的修改需要新的510(k)許可或上市前批准申請,我們可能無法及時或根本無法獲得修改或額外適應症的額外許可或批准。獲得許可和批准可能是一個耗時的過程,延遲獲得所需的未來許可或批准將對我們及時推出新產品或增強產品的能力產生不利影響,這反過來又會損害我們未來的增長。
我們未來臨床試驗的結果可能不支持我們的候選產品主張,或者可能導致發現不良副作用。
我們還沒有完成任何臨床試驗,我們不能確定他們的結果是否會支持我們的候選產品聲明,或者FDA是否會同意我們的結論。臨床前研究和早期臨床試驗的成功並不能確保後來的臨床試驗將會成功,我們也不能確定後來的試驗會複製以前的試驗和臨床前研究的結果。臨床試驗過程可能無法證明我們的候選產品對於建議的指定用途是安全有效的,這可能會導致我們放棄某個候選產品,並可能延誤其他候選產品的開發。我們臨床試驗的任何延遲或終止都將推遲我們提交的產品申請,並最終推遲我們將候選產品商業化並創造收入的能力。參加臨床試驗的患者也有可能會經歷目前不在候選產品簡介中的不良副作用。
我們計劃在美國境外進行初步概念驗證試驗,並將該試驗的相關數據提交給FDA,以儘量減少美國批准的臨床要求。無法保證FDA會接受這些數據。
人體試驗通常設計爲從概念驗證試驗開始,然後進入「臨時」或批准試驗。我們打算在美國境外進行概念驗證試驗。完成後,我們計劃在提交前會議上向FDA提交這項研究的相關數據,以請求「突破狀態」,以儘量減少美國批准的臨床要求。第一項試驗的目的並不是取代FDA爲支持我們在美國批准提交的申請而要求的試驗,而是可能影響該試驗的規模。無法保證FDA會接受我們國際試驗的數據,也無法保證他們不會要求我們進行額外的研究來補充本試驗。我們需要進行的任何額外試驗都將是昂貴且耗時的,並且可能需要我們籌集額外的資金,但我們對此沒有承諾。
我們的臨床研究可能會被推遲或受到許多因素的不利影響,包括招募患者的困難。
臨床測試可能成本高昂,耗時多年,結果不確定,容易受到不同解釋的影響。此外,臨床前和早期臨床研究的成功並不能確保大規模研究的成功或預測最終結果。早期研究中可接受的結果可能不能在以後的研究中複製。一些公司在高級臨床研究方面遭遇了重大挫折,即使在早期的研究中取得了令人振奮的結果。臨床研究期間的陰性或不確定的結果或不良事件或事件可能會導致臨床研究重新進行或終止。此外,未能適當地構建臨床研究可能會導致不良事件或事件的發生率很高,這可能會導致臨床研究暫停、重做或終止。我們研究中的第三方參與者未能或未能遵守他們遵守協議和/或法律要求的義務,也可能導致我們無法在提交給監管機構的報告中使用受影響的數據。
臨床研究的及時完成取決於我們招募足夠數量的患者直至研究結束的能力。由於多種原因,我們在臨床研究中招募患者時可能會遇到困難,包括:
|
● |
正在調查的疾病的嚴重程度; |
|
● |
患者人群的規模和性質有限; |
|
● |
我們的方案和其他臨床研究方案中定義的患者資格標準; |
|
● |
研究方案的性質,包括入組受試者接受的治療的吸引力或相關的不適和風險; |
|
● |
未來大流行可能導致臨床研究出現困難和延誤; |
|
● |
在臨床研究地點獲得機構審查委員會(「IRS」)批准的能力; |
|
● |
臨床醫生和患者對我們的產品相對於其他可用療法的潛在優點、缺點和副作用的看法,包括可能被批准用於我們正在追求的適應症的任何新藥或治療方法; |
|
● |
由於方案限制,參加產品臨床研究可能會限制患者參加未來其他療法臨床研究的能力的可能性或看法; |
|
● |
我們的軟件不夠安全,無法維護患者隱私的可能性或感覺; |
|
● |
在治療期間和治療後充分監測患者的能力; |
|
● |
適當的臨床研究研究人員、支持人員、藥物和其他治療用品的可用性以及患者與臨床中心的距離; |
|
● |
醫生或我們獲得和維持患者同意的能力;以及 |
|
● |
參加臨床研究的患者選擇退出或無法完成臨床研究的風險。 |
如果我們難以招募和保留足夠數量或多樣性的患者來按計劃進行臨床研究,或者遇到其他困難,我們可能需要推遲、終止或修改正在進行的或計劃中的臨床研究,其中任何一項都會對我們的業務產生不利影響。
即使我們的產品已獲得FDA批准或批准,如果我們或我們的供應商未能遵守FDA的持續要求,或者如果我們的產品遇到意外問題,這些產品也可能會受到限制或退出市場。
我們獲得批准或批准的任何產品,以及該產品的製造流程、報告要求、批准後的臨床數據和促銷活動,都將受到FDA和其他國內外監管機構的持續監管審查、監督和定期檢查。特別是,我們和我們的供應商將被要求遵守FDA的質量體系法規或QSR,該法規涵蓋了我們獲得批准或批准的任何產品的設計、測試、生產、控制、質量保證、標籤、包裝、儲存和運輸的方法和文檔。FDA通過定期檢查來執行QSR和其他法規。如果我們或我們的供應商未能遵守FDA實施的適用法規,或未能對任何不利的檢查意見或產品安全問題作出及時和充分的回應,除其他事項外,可能會導致下列任何執法行動:
|
● |
無標題信件、警告信、罰款、禁令、同意法令和民事處罰; |
|
● |
應對或辯護此類行動的意外支出; |
|
● |
客戶通知維修、更換、退款; |
|
● |
召回、扣留或扣押我們的產品; |
|
● |
限產、部分停產、全面停產的; |
|
● |
拒絕或推遲我們對新產品或改良產品的上市前許可或上市前批准的請求; |
|
● |
經營限制; |
|
● |
撤回對已授予的PMA批准的上市前許可; |
|
● |
拒絕批准我公司產品出口的; |
|
● |
刑事起訴。 |
如果發生任何這些行爲,都會損害我們的聲譽,導致我們的產品銷售和盈利能力受到影響,並可能阻止我們產生收入。此外,我們的關鍵零部件供應商可能不遵守所有適用的監管要求,這可能導致我們無法及時並按所需數量生產產品(如果有的話)。
即使產品獲得了監管許可或批准,此類許可或批准也可能受到產品銷售預期用途的限制,並降低我們成功商業化產品和從產品中產生收入的潛力。如果FDA確定我們的宣傳材料、標籤、培訓或其他營銷或教育活動構成對未經批准的使用的宣傳,則可以要求我們停止或修改我們的培訓或宣傳材料,或對我們採取監管執法行動。如果其他聯邦、州或外國執法當局認爲我們的培訓或其他宣傳材料構成未經批准的使用的宣傳,也可能會採取行動,這可能會導致其他法定當局的巨額罰款或處罰,例如禁止虛假索賠報銷的法律。
此外,我們可能被要求進行昂貴的上市後測試和監控,以監控我們產品的安全性或有效性,並且我們必須遵守醫療器械報告要求,包括報告與我們產品相關的不良事件和故障。我們的產品在以後發現以前未知的問題,包括意想不到的不良事件或意料之外的嚴重或頻率的不良事件、製造問題,或未能遵守監管要求(如QSR),可能會導致標籤更改、對此類產品或製造工藝的限制、產品從市場上撤回、自願或強制召回、要求維修、更換或退還我們製造或分銷的任何醫療器械的成本、罰款、暫停監管批准、產品扣押、禁令或施加民事或刑事處罰,從而對我們的業務、經營業績和前景產生不利影響。
我們的產品未來可能會被產品召回,這可能會損害我們的聲譽、業務和財務業績。
FDA和類似的外國政府當局有權要求在設計或製造中存在重大缺陷或缺陷的情況下召回商業化產品。在FDA的情況下,要求召回的權力必須基於FDA發現該設備有合理的可能性會導致嚴重傷害或死亡。如果在設備中發現任何重大缺陷,製造商可以主動召回產品。由於組件故障、製造錯誤、設計或標籤缺陷或其他缺陷和問題,我們或我們的某個經銷商可能會發生政府強制或自願召回。召回我們的任何產品都會轉移管理和財務資源,並對我們的財務狀況和運營結果產生不利影響。FDA要求某些類別的召回在召回開始後10個工作日內向FDA報告。公司被要求保留某些召回記錄,即使這些召回不需要向FDA報告。我們可能會在未來啓動涉及我們產品的自願召回,我們認爲這些召回不需要通知FDA。如果FDA不同意我們的決定,他們可以要求我們將這些行爲報告爲召回。未來的召回聲明可能會損害我們在客戶中的聲譽,並對我們的銷售產生負面影響。此外,FDA可能會對召回時沒有報告召回情況採取執法行動。
如果我們的產品導致或導致死亡或重傷,或以某些方式發生故障,我們將遵守醫療器械報告法規,這可能會導致自願糾正行動或機構執法行動。
根據FDA醫療器械報告法規,醫療器械製造商必須向FDA報告設備已經或可能已經導致或促成死亡或嚴重傷害的信息,或者如果設備或我們的類似設備的故障再次發生,則可能導致或促成死亡或嚴重傷害的故障。如果我們未能在規定的時間內向FDA報告這些事件,或者根本沒有報告這些事件,FDA可能會對我們採取執法行動。涉及我們產品的任何此類不良事件也可能導致未來的自願糾正行動,例如召回或客戶通知,或機構行動,例如檢查或執法行動。任何糾正行動,無論是自願還是非自願,以及在訴訟中爲自己辯護,都需要我們投入時間和資本,分散管理層對我們業務運營的注意力,並可能損害我們的聲譽和財務業績。
用於製造我們設備的某些零部件可能會出現全球供應短缺的情況,這可能會影響我們爲客戶製造設備或維持研發時間表的能力。
我們設備的製造中使用了許多零部件,許多製造商將其用於各種產品。我們將與其他製造商競爭這些零部件的供應。此外,我們目前設計中的某些零件可能會被我們的供應商停產,要求我們尋找替代零件。此問題可能需要我們更改設備的設計或購買這些零件的大量庫存,以防止製造延誤。設計變更可能需要FDA批准。我們可能無法採購替代零部件或足夠的原材料庫存,從而導致無法生產我們的設備。
美國立法或FDA監管改革可能會使我們獲得候選產品的監管批准以及獲得批准後製造、營銷和分銷我們的產品變得更加困難和成本。
國會不時起草並提出立法,可能會顯着改變管理監管批准、受監管產品的製造和營銷或其報銷的法定條款。此外,FDA經常以可能顯着影響我們的業務和產品的方式修訂或重新解釋FDA法規和指南。任何新法規或對現有法規的修訂或重新解釋都可能會帶來額外成本或延長未來產品的審查時間。此外,FDA法規和指南經常被該機構修改或重新解釋,這可能會對我們的業務和產品產生重大影響。無法預測是否會頒佈立法變更,或者FDA法規、指南或解釋是否會發生變化,以及這些變化(如果有的話)可能會產生什麼影響。
由於我們業務的專業性,終止與我們的關鍵員工、顧問和顧問的關係可能會阻止我們成功運營我們的業務,包括開發我們的產品、進行臨床研究、將我們的產品商業化和獲得任何必要的融資.
我們高度依賴執行團隊的成員,他們的服務的流失可能會對我們目標的實現產生不利影響。雖然我們已與每位關鍵高管簽訂了就業或諮詢協議,但他們中的任何人都可以隨時離職。我們沒有爲任何員工購買「關鍵人員」保險。我們一名或多名現有員工的服務流失可能會阻礙我們實現業務目標。
醫療器械領域的人才競爭非常激烈,我們在很大程度上依賴於我們吸引和留住合格科技和管理人才的能力。我們未來的成功取決於我們吸引、留住和激勵高技能員工的能力。爲了成功地將我們的產品商業化,我們將被要求擴大我們的員工隊伍,特別是在研發和臨床研究、財務、會計和報告、銷售和營銷以及供應鏈管理領域。這些活動將需要增加新的人員,並由現有的管理人員發展更多的專門知識。我們面臨着來自衆多製藥、生物製藥和生物技術公司以及學術和其他研究機構對合格人才的激烈競爭。我們可能無法以可接受的條件吸引和留住這些人,甚至根本不能。如果不這樣做,可能會對我們的業務造成實質性的損害。
未能從第三方付款人獲得和維持足夠的保險範圍和報銷可能會對我們產品(如果獲得批准)的接受產生不利影響,並減少我們的收入。
假設我們的產品獲得批准,我們預計我們的絕大多數收入將來自第三方付款人,要麼直接在我們計劃向患者提供候選設備的市場向我們支付,要麼通過向醫院或其他實體付款間接支付,這些實體可能會在未來向患者提供我們的候選設備。
在美國,私人付款人覆蓋了人口的最大部分,其餘人要麼沒有保險,要麼由政府付款人覆蓋。美國境外的大多數第三方付款人是政府機構、政府贊助的實體或其他在國家或地區政府嚴格監管要求下運營的付款人。
第三方付款人可能會拒絕支付和報銷某些程序、用品或服務。此外,一些第三方付款人可能會拒絕爲特定患者承保和報銷我們的產品,即使付款人制定了針對我們產品或之前批准的報銷我們產品的優惠承保政策。此外,私人和政府付款人在批准保險範圍或確定治療報銷時可能會考慮治療費用。
世界各地的私人和政府付款人越來越多地挑戰醫療產品和服務的價格。此外,控制醫療成本已成爲世界各國政府的首要任務。採取額外的價格控制和成本控制措施,以及在現有控制和措施的司法管轄區採取更具限制性的政策,可能會進一步限制我們的收入和經營業績。如果第三方付款人不認爲我們的產品或我們的產品與額外治療的組合在所需的成本測試模型下具有成本合理性,他們可能不會爲其人群承保我們的產品,或者如果他們這樣做,報銷水平可能不足以讓我們能夠以盈利的方式銷售我們的產品。
世界各地醫療設備患者治療的報銷由每個國家在國家或國家以下各級建立的複雜機制管理。這些機制在各國之間差異很大,可能是非正式的,有些不可預測的,並且不斷演變,反映了這些國家減少醫療保健公共支出的努力。因此,在全球範圍內獲得和維持醫療器械患者治療的報銷變得更具挑戰性。我們無法保證我們產品的使用將獲得報銷批准,也無法保證我們現有的報銷批准將在任何國家/地區維持。
我們未能確保或維持美國第三方付款人爲我們的產品提供足夠的保險或報銷,或在我們銷售產品的其他司法管轄區,可能會對我們的業務、收入和運營業績產生重大不利影響。
我們可能無法成功確保和維護必要的報銷代碼,以促進準確、及時地爲我們的產品或我們產品的醫生服務計費.
第三方付款人、醫療保健系統、政府機構或其他團體經常發佈報銷代碼,以方便對醫療保健提供中使用的產品和醫生服務計費。在美國境內,與我們的產品最直接相關的計費代碼包含在醫療保健通用程序編碼系統(「HCPCS代碼集」)中。HCPCS代碼集包含描述醫生服務的I級代碼,也稱爲通用程序術語代碼(「CPt代碼」)和主要描述產品的II級代碼。醫療保險和醫療補助服務中心(「CMS」)負責發佈HCPCS II級代碼。美國醫學會發布HCPCS I級代碼。
目前不存在HCPCS代碼或CPt代碼來描述與使用我們的產品提供治療相關的醫生服務。我們可能無法確保與我們產品相關的醫生服務的HCPCS代碼和CPt代碼。我們未來的收入和結果可能會受到CPt代碼的缺乏的影響,因爲當無法確定是否可以獲得足夠的報銷以支付向患者提供治療所需的時間、精力、技能、執業費用和醫療事故費用時,醫生可能不太可能開處方治療。
在美國以外,我們尚未獲得代碼來描述我們的產品或記錄與使用我們的產品提供治療相關的醫生服務。未能獲取和維護這些代碼可能會影響我們業務的未來增長。
如果我們無法與醫生建立良好的關係,我們的業務可能會受到負面影響。
我們的業務模式要求我們與擁有大量患者來源的醫生建立和維持良好的關係,這將爲醫生和公司創造治療收入。如果我們無法與醫生建立良好的關係並維持他們,將危及我們未來產生收入的能力。
無法保證Medicare或Medicare行政承包商將爲我們的產品提供保險或足夠的付款率。
我們預計,使用我們產品的很大一部分患者將成爲醫療保險收費服務計劃的受益人。未能獲得或維持醫療保險的覆蓋範圍或維持足夠的報銷將減少我們的收入,還可能影響美國和其他地區其他第三方付款人的覆蓋範圍和報銷決定。
醫療保險可能會將我們的醫療設備歸類爲耐用醫療設備(「DMS」)。醫療保險有權發佈全國保險決定或將保險決定推遲給其區域醫療保險行政承包商(「MAC」)。只有兩個MAC負責管理整個DMS計劃,這一事實可能會對我們向MAC個人醫療政策決策者申請保險的能力產生負面影響。醫療保險或DMS MAC缺乏積極的覆蓋範圍確定或未來對現有覆蓋範圍的限制將對我們未來的收入產生重大影響。
此外,Medicare還有權公佈DMS產品的報銷金額。Medicare未來可能會公佈我們產品的報銷金額,但不會反映我們產品當時的價格。美國和世界各地的私人付款人經常參考醫療保險費用表。醫療保險公佈的產品報銷金額低於我們產品的既定價格可能會大幅減少我們在美國和我們其他活躍市場的非醫療保險付款人的收入和經營業績。
即使我們的產品獲得了Medicare的授權,CMS也需要對某些DMS項目進行事先授權。在向受益人提供之前未獲得事先授權的此類物品的索賠將自動被拒絕。如果醫療保險將我們的一種產品添加到需要事先授權的物品列表中,我們爲原本可以在醫療保險收費服務計劃下使用我們產品的患者計費和獲得報銷的能力可能會降低。
我們無法保證我們可以爲我們的產品獲得過渡性、快速或擴大的醫療保險覆蓋範圍。CMS預計將發佈有關新興技術覆蓋範圍的規則;然而,沒有有關預期規則內容的具體信息,我們無法保證任何有關新興技術的新規則將適用於我們未來的產品。
與我們的知識產權和信息技術相關的風險
如果第三方計算機系統故障、第三方系統遭受網絡攻擊或網絡安全缺陷,我們的業務和運營將受到影響。
我們依靠信息技術(「IT」)系統,包括第三方「基於雲」的服務提供商,來保存財務記錄、維護實驗室數據、臨床數據和公司記錄,與員工和外部各方進行溝通,以及運行其他關鍵功能。這包括電子郵件、其他通信工具、電子文件儲存庫和檔案等關鍵系統。如果這些第三方信息技術提供商中的任何一個因計算機病毒、未經授權的訪問、惡意軟件、自然災害、火災、恐怖主義、戰爭和電信故障、電氣故障、網絡攻擊或互聯網上的網絡入侵而受到威脅,那麼敏感的電子郵件或文件可能會被曝光或刪除。同樣,如果我們對互聯網的訪問受到威脅,並且我們無法與第三方it提供商連接,我們可能會導致業務中斷。隨着來自世界各地的未遂攻擊和入侵的數量、強度和複雜性增加,安全漏洞或破壞的風險普遍增加,特別是通過網絡攻擊或網絡入侵,包括計算機黑客、外國政府和網絡恐怖分子。此外,我們依賴這些第三方來保護有關我們參加臨床試驗的員工和受試者的重要機密個人數據。如果發生中斷事件並導致第三方it提供商的運營中斷,可能會導致我們的藥物開發計劃中斷。例如,已完成、正在進行或計劃中的臨床試驗中的臨床試驗數據丟失可能會導致我們的監管審批工作延遲,並顯著增加我們恢復或複製數據的成本。如果任何中斷或安全漏洞導致我們的數據或應用程序丟失或損壞,或者不適當地披露機密或專有信息,我們可能會招致責任,我們候選產品的開發可能會延遲或可能失敗。
人工智能帶來了可能影響我們業務的風險和挑戰,包括對我們的機密信息、專有信息和個人數據構成安全風險。
人工智能的開發和使用中的問題,再加上不確定的監管環境,可能會對我們的業務運營造成聲譽損害、責任或其他不利後果。與許多技術創新一樣,人工智能帶來的風險和挑戰可能會影響我們的業務。對於經過法律和信息安全審查的特定用例,我們可能會採用生成性人工智能工具並將其集成到我們的系統中。我們的供應商可能會在不向我們披露其使用情況的情況下,將生成性人工智能工具整合到其產品中,並且這些生成性人工智能工具的提供商可能無法滿足有關隱私和數據保護的現有或快速演變的法規或行業標準,並可能會抑制我們或我們的供應商維持足夠水平的服務和體驗的能力。如果我們、我們的供應商或我們的第三方合作伙伴因使用生成性人工智能而經歷實際或預期的違規或隱私或安全事件,我們可能會丟失寶貴的知識產權和機密信息,我們的聲譽和公衆對我們安全措施有效性的看法可能會受到損害。此外,世界各地的不良行爲者使用越來越複雜的方法,包括使用人工智能,從事涉及盜竊和濫用個人信息、機密信息和知識產權的非法活動。這些結果中的任何一個都可能損害我們的聲譽,導致寶貴的財產和信息損失,並對我們的業務造成不利影響。
網絡安全風險和網絡事件可能會對我們的業務產生不利影響並擾亂運營。
網絡事件可能是故意攻擊或無意事件造成的。這些事件可能包括但不限於未經授權訪問數字系統,目的是挪用資產或敏感信息、損壞數據或造成運營中斷。這些事件的結果可能包括但不限於:中斷運營、錯誤陳述財務數據、被盜資產或信息的責任、網絡安全保護成本增加、訴訟和聲譽損害對客戶或投資者信心造成不利影響。我們正在實施各種制度和進程,以側重於識別、預防、緩解和解決問題。然而,這些措施不能提供絕對的安全性,我們的系統可能容易受到網絡安全漏洞的攻擊,例如病毒、黑客攻擊和來自未經授權的入侵的類似中斷。此外,我們依賴第三方服務提供商提供某些服務,如工資和稅務服務。我們的系統或第三方系統的任何故障都可能危及我們的敏感信息和/或員工的個人身份信息或受HIPAA保密要求約束的患者健康信息。雖然我們正在確保網絡保險的過程中,可能涵蓋與網絡事件相關的某些風險,但不能保證保險將足以覆蓋任何此類責任。
我們可能會因與專利和其他知識產權有關的訴訟或其他程序而產生大量費用。
當我們確定有可能取得成功並可能導致知識產權增值時,我們可能會不時尋求對侵權者行使我們的知識產權。如果我們選擇對某一方強制執行我們的專利權,那麼該個人或公司有權要求法院裁定此類專利無效或不應強制執行。此外,如果在法定適用時間內向美國專利商標局(USPTO)提交授權後程序(如各方間審查和授權後審查)的請願書,我們的專利和我們許可的專利的有效性可能會受到質疑。這些訴訟和訴訟是昂貴的,會消耗時間和資源,並轉移管理和科學人員的注意力,即使我們成功地阻止了對此類專利的侵權。此外,還有一種風險,即法院將裁定此類專利無效,我們無權阻止對方使用這些發明。還有一種風險是,即使這些專利的有效性得到支持,法院也會以對方的活動沒有侵犯我們的知識產權爲理由,拒絕阻止對方。此外,近年來,美國最高法院修改了美國專利商標局在過去20年中授予專利時使用的一些測試,這可能會降低我們能夠獲得專利的可能性,並增加我們獲得或許可的任何專利受到挑戰的可能性。
如果第三方聲稱我們的產品侵犯了他們的知識產權,我們可能被迫花費大量財務資源和管理時間來抵禦此類行爲,我們的財務狀況和運營業績可能會受到影響。
第三方可能會聲稱我們的產品侵犯了他們的專利和其他知識產權。確定第三方專利權可能特別困難,因爲一般而言,專利申請可以在其最早優先權日期之後的較長時間內保密。從歷史上看,在醫療器械和相關行業中,涉及專利和其他知識產權的訴訟數量很大。如果競爭對手挑戰我們的專利或其他知識產權,或聲稱我們的產品侵犯了我們的專利或其他知識產權,我們可能會招致巨額訴訟費用,被迫對我們的產品設計進行昂貴的更改,支付特許權使用費或其他費用以繼續製造和銷售我們的產品,或支付巨額損害賠償。第三方侵權索賠,無論結果如何,不僅會消耗我們的財力,還會分散我們管理層的時間和精力。
如果我們無法保護我們產品中使用的知識產權,其他人可能會複製我們的創新,這可能會損害我們在市場中有效競爭的能力。
我們專利的實力涉及複雜的法律和科學問題,並且可能是不確定的。這些專利申請可能會受到質疑或未能獲得已頒發的專利,或者如果已頒發,這些專利和我們現有的專利可能過於狹窄,無法阻止第三方圍繞我們的知識產權進行開發或設計,在這種情況下,我們可能會失去競爭優勢,這可能會導致我們的業務受到損害。
我們可能會受到指控,稱我們的員工錯誤地使用或披露了其前僱主據稱的商業機密。
正如醫療器械行業的常見情況一樣,我們可能會僱用以前在其他醫療器械公司(包括我們的競爭對手或潛在競爭對手)僱用的個人。我們可能會聲稱這些員工或我們使用或披露了其前僱主的商業祕密或其他專有信息。可能需要提起訴訟來抵禦這些索賠。即使我們成功地對這些索賠進行了辯護,訴訟也可能會導致巨額成本並分散管理層的注意力。
如果我們不能保護我們的專有信息和專有技術的機密性,我們的技術和產品的價值可能會受到不利影響。
除專利技術外,我們還依賴非專利專有技術、工藝、商業祕密和專有技術。任何非自願向第三方披露或盜用我們的機密或專有信息都可能使競爭對手複製或超過我們的技術成就,從而潛在地侵蝕我們在市場上的競爭地位。我們尋求通過與員工、顧問和第三方簽訂保密協議來部分保護機密或專有信息。雖然我們要求我們的所有員工、顧問、顧問和任何能夠訪問我們專有技術、信息和技術的第三方簽訂保密協議,但我們不能確定這些技術、信息和技術不會被披露,或者競爭對手不會以其他方式獲得我們的商業祕密或獨立開發基本上相同的信息和技術。這些協議可能被終止或被違反,我們可能沒有足夠的補救措施來終止或違反任何此類協議。此外,在未經授權使用或披露的情況下,這些協議可能無法爲我們的商業祕密和專有技術提供有意義的保護。如果我們的任何員工以前受僱於其他製藥、醫療技術或生物技術公司,這些僱主可能會爲我們指控他們的醫療器械開發活動違反了商業祕密和其他類似的索賠。
與此次發行相關的風險
在我們和我們的核數師完成之前,對截至2024年9月30日止期間的業績進行初步估計存在重大限制’ 該時期的財務審查程序。我們的獨立註冊會計師事務所尚未對初步未經審計的結果進行審計或審查,也不對初步未經審計的結果發表意見或任何其他形式的保證。我們或我們的獨立註冊會計師事務所可能會發現需要我們對持續經營業務的營業虧損和所得稅前淨虧損的初步估計進行調整的項目。
「招股說明書摘要-第二季度初步業績(未經審計)」中包含的初步現金流量估計並不是我們截至2024年9月30日期間的現金流量的全面報表。我們截至2024年9月30日的財務報表將在本次發售完成後才能提供,因此,您在投資此次發售之前將無法獲得。我們截至2024年9月30日的實際結果可能與我們提供的初步估計大不相同,這是因爲我們完成了財務結算程序、最終調整以及從現在到我們這些時期的財務結果最終確定之間的其他事態發展。你不應該過分依賴初步估計,初步估計不一定代表未來預期的結果。本文件所載的初步財務數據由管理層編制,並由管理層負責。我們的獨立註冊會計師事務所Forvis-Mazars LLP尚未對此類初步估計進行審計、審查、編制或執行任何程序。因此,Forvis和Mazars LLP不對此發表意見或任何其他形式的保證。
We have broad discretion in how we use the proceeds of this offering and may not use these proceeds effectively, which could affect our results of operations and cause our common stock to decline.
We will have considerable discretion in the application of the net proceeds of this offering. We intend to use the net proceeds from this offering primarily to fund our clinical trial, for other research and development, and for working capital. As a result, investors will be relying upon management’s judgment with only limited information about our specific intentions for the use of the net proceeds of this offering. We may use the net proceeds for purposes that do not yield a significant return or any return at all for our stockholders. In addition, pending their use, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
We will require substantial funding, which may not be available to us on acceptable terms, or at all, and, if not so available, may require us to delay, limit, reduce or cease our operations.
We are using the proceeds from this offering to, among other uses, advance our lead product candidate toward commercial launch. Developing medical device products, including conducting clinical trials, is expensive. We will require substantial additional future capital in order to complete clinical development and commercialize our product candidate. If the FDA requires that we perform additional studies or clinical trials, our expenses would further increase beyond what we currently expect and the anticipated timing of any potential clearance of our product candidate would likely be delayed. Further, there can be no assurance that the costs we will need to incur to obtain regulatory approval will not increase.
我們將繼續需要大量額外資金來繼續我們的臨床開發和商業化活動。由於我們候選產品的成功開發尚不確定,因此我們無法估計完成研發並將正在開發的產品商業化所需的實際資金金額。
我們估計,我們將需要約4000萬美元的額外融資來爲我們的運營提供資金。我們相信,我們現有的現金和現金等值物加上此次發行的收益將足以滿足我們直到2025年第二季度(但不超過2025年第二季度)的預計運營需求。此類預測取決於我們內部資助的臨床前和臨床活動的變化,包括計劃外的臨床前和臨床活動。臨床試驗的時間和成本很難預測,因此上述估計可能被證明是不準確的。我們沒有承諾提供此類額外所需融資,並且可能會被要求通過出售額外股權或債務證券來籌集此類融資。
我們未來資金需求的金額和時間將取決於許多因素,包括但不限於:
|
● |
我們的臨床試驗計劃是否會及時完成; |
|
|
● |
我們臨床試驗的進展、成本、結果和時間; |
|
|
● |
尋求和獲得FDA許可和任何其他監管批准的結果、成本和時間; |
|
|
● |
與確保和建立商業化和製造能力相關的成本; |
|
|
● |
市場對我們候選產品的認可度; |
|
|
● |
收購、許可或投資企業、產品、候選產品和技術的成本; |
|
|
● |
我們維持、擴大和執行知識產權組合範圍的能力,包括我們可能被要求支付或可能收到的與任何專利或其他知識產權的許可、提交、起訴、辯護和執行有關的任何付款的金額和時間; |
|
|
● |
我們需要和有能力聘請更多的管理人員以及科學和醫療人員; |
|
|
● |
競爭候選產品和新產品批准的影響;以及 |
|
|
● |
我們需要實施更多的內部系統和基礎設施,包括財務和報告系統。 |
Some of these factors are outside of our control. We may seek additional funding through a combination of equity offerings, debt financings, government or other third-party funding, commercialization, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements. Additional funding may not be available to us on acceptable terms or at all. In addition, the terms of any financing may adversely affect the holdings or the rights of our stockholders.
If we are unable to obtain funding on a timely basis, we may be required to significantly curtail one or more of our research or development programs. We also could be required to seek funds through arrangements with collaborative partners or otherwise that may require us to relinquish rights to some of our technologies or product candidates or otherwise agree to terms unfavorable to us.
此次發行的買家將立即經歷淨有形淨資產的大幅稀釋。
本次發售生效後,每股公開發售價格將大幅高於我們普通股的調整後每股有形賬面淨值的預計水平。假設我們的普通股以每股14.31美元的假定公開發行價出售698,812股,即我們普通股於2024年10月31日在納斯達克的收盤價,在扣除承銷折扣和佣金以及估計應支付的發售費用後,您將產生形式上的立即攤薄,調整後的有形賬面淨值約爲每股6.6美元。由於在此次發行中購買證券的投資者被稀釋,如果我們的公司發生清算,投資者獲得的收益可能會大大低於在此次發行中支付的購買價格。有關您參與此次發售將產生的攤薄的更詳細討論,請參閱下面標題爲「攤薄」的部分。只要股份是根據已發行期權、認股權證或可轉換票據以低於本次發行中我們普通股的公開發行價的行使或轉換價格發行的,您將產生進一步的攤薄。
如果增發股本以籌集資本、爲收購融資或與戰略交易相關,您的所有權可能會被稀釋。
我們將需要額外的大量資金,以便將我們的候選產品商業化。我們打算尋求爲我們的業務籌集額外的資金,爲收購提供資金,或通過發行股本或可轉換債務證券來發展戰略關係,這將減少我們現有股東的持股比例。我們的董事會有權在不採取行動或股東投票的情況下,發行我們授權但未發行的全部或部分普通股或優先股。我們的公司證書授權我們發行最多500,000,000股普通股和10,000,000股優先股。未來發行普通股或優先股將減少你對股東投票事項的影響力,並將稀釋每股收益。此外,任何新發行的優先股都可以享有優先於普通股的權利、優先和特權。這些權利、優惠和特權可以包括,除其他外,在宣佈或向我們普通股持有人支付股息或其他分配或規定優先清算權之前必須支付的股息。這些權利、優惠和特權可能會對我們普通股持有者的權利產生負面影響,以及將這些優先股轉換爲我們普通股的權利,其速度或價格將對我們普通股的流通股產生稀釋影響。
如果我們的股價在上市後出現波動,您的投資可能會損失很大一部分。
我們普通股的市場價格可能會因本招股說明書中描述的風險因素以及其他我們無法控制的因素而受到廣泛波動的影響,例如投資者認爲與我們相當的公司估值的波動。此外,股票市場經歷了價格和成交量的波動,這些波動已經並將繼續影響許多公司的股權證券的市場價格。這些波動往往與這些公司的經營業績無關或不成比例。這些廣泛的市場和行業波動,以及一般的經濟、政治和市場狀況,如經濟衰退、利率變化或國際貨幣波動,可能會對我們普通股的市場價格產生負面影響。過去,許多經歷過股票市場價格波動的公司都會受到證券集體訴訟的影響。我們未來可能會成爲這類訴訟的目標。針對我們的證券訴訟可能導致巨額成本,並將我們管理層的注意力從其他業務上轉移,這可能會嚴重損害我們的業務。
本次發行可能會導致我們普通股的交易價格下跌。
每股價格,加上本次發行完成後我們發行的普通股股數,可能會導致我們普通股的市場價格立即下跌。本次發行完成後,這種下降可能會繼續。
我們從未爲我們的股本支付過股息,我們預計在可預見的未來也不會支付股息。
我們從未對任何股本支付股息,目前打算保留任何未來收益來資助我們的業務增長。我們未來還可能達成信貸協議或其他借款安排,這將限制我們宣佈或支付普通股現金股息的能力。未來支付股息的任何決定將由董事會酌情決定,並取決於我們的財務狀況、經營業績、資本要求、一般業務狀況以及董事會可能認爲相關的其他因素。因此,在可預見的未來,證券的資本增值(如果有的話)將是唯一的收益來源(如果有的話)。
與我們普通股相關的風險
我們於2024年10月24日完成了反向股票分拆,以重新遵守納斯達克上市規則,我們無法預測這種反向股票分拆將對我們普通股股票的市場價格產生的影響。
我們的董事會批准了我們普通股的1比20反向股票拆分,該計劃於東部時間2024年10月24日晚上11:59生效。我們無法預測反向股票分拆將對我們普通股股票的市場價格產生的影響,並且在類似情況下公司類似反向股票分拆的歷史各不相同。一些投資者可能對反向股票分拆持負面看法。即使反向股票分割對我們普通股股票的市場價格、我們的業務表現和財務業績、總體經濟狀況和市場對我們業務的看法產生積極影響,以及我們可能無法控制的其他不利因素也可能導致我們普通股價格下降。反向股票分割後。
此外,即使反向股票拆分確實導致我們普通股的每股市場價格上漲,反向股票拆分後的每股市場價格也可能不會與實施反向股票拆分前我們已發行普通股股數的減少成比例地上漲。因此,即使每股市場價格上漲,反向股票分拆後我們普通股的總市值也可能低於反向股票分拆前的總市值。此外,即使反向股票分拆後我們普通股的每股市場價格最初上漲,市場價格也不會保持在該水平。
如果我們普通股的市場價格在反向股票分拆後下跌,那麼作爲絕對數字和佔我們總市值的百分比的跌幅可能會大於在沒有反向股票分拆的情況下,由於我們普通股市場的流動性下降。因此,反向股票分拆後我們普通股的總市值可能低於反向股票分拆前的總市值。
本次發行中提供的普通股或預融資股沒有公開市場。
本次發行中提供的普通股或預融資股沒有公開交易市場,我們預計不會發展市場。此外,我們無意申請在任何證券交易所或國家認可的交易系統(包括納斯達克)上市普通股或預融資股。如果沒有活躍的市場,普通股和預融資股的流動性將會受到限制。
在本次發行中購買的普通股和/或預融資憑證的持有人作爲我們普通股持有人,對於此類普通股或預融資憑證的普通股股份沒有任何權利,直到此類持有人行使其普通股或預融資憑證並收購我們的普通股。
除普通股和預融資股中規定的情況外,在普通股或預融資股持有人因普通股或預融資股行使而收購我們的普通股股份之前,普通股或預融資股持有人將對我們的普通股股份沒有任何權利,包括投票權,有關股息的權利,請參閱「我們提供的證券描述」。行使普通股或預融資證後,持有人僅有權就記錄日期發生在行使日期之後的事項行使我們普通股持有人的權利。
A系列令的條款可能會阻止第三方收購我們。
A系列認購證規定,如果發生「基本交易」,(如相關授權協議中的定義,通常包括與另一個實體的任何合併、將我們的全部或絕大部分資產出售、轉讓或以其他方式處置給另一個實體,或一個人收購我們50%以上的普通股),每位A系列令狀持有人將有權在基本交易完成之前的任何時候要求我們以等於布萊克-斯科爾斯價值的現金購買價格回購普通令狀(根據認購證協議計算)該系列A證當時剩餘未行使部分的金額,這可能會對我們的財務狀況和/或運營結果產生重大不利影響,並可能阻止或阻止第三方收購我們。
我們普通股的所有權集中在我們現有的高管和董事手中可能會阻止新投資者影響重大公司決策。
我們的執行官和董事及其附屬公司(即我們的主要股東)總共擁有我們在此次發行之前約30.7%的已發行普通股。因此,這些人共同行動,將能夠對所有需要股東批准的事項產生重大影響,包括董事的選舉和罷免、任何合併、合併、出售我們所有或幾乎所有資產,或其他重大公司交易。少數股東無法推翻我們主要股東的決定。這種控制水平也可能對我們股票的市值產生不利影響,因爲我們的主要股東可能會制定或實施導致損失的交易、政策或計劃,並且可能不會採取任何措施來提高我們在金融界的知名度和/或可能出售足夠數量的股票以顯着降低我們的每股價格。
未來發行我們的普通股基礎憑證和可轉換票據可能會導致我們普通股的市場價格下跌,並導致您持有的股份被稀釋。
截至本招股說明書之日,我們有未行使的認購權,可按每股8.26美元的加權平均行使價購買112,534股我們的基礎普通股;以及未行使的可轉換票據,可按40.00美元的平均換股價購買33,250股我們的普通股。未來我們普通股相關可轉換證券的發行可能會導致我們普通股的市場價格下跌。我們無法預測未來發行證券對我們普通股價格的影響(如果有的話)。無論如何,未來發行我們的股票將導致您所持股份的稀釋。此外,認爲我們可能會發行新的證券,可能會對我們普通股的市場價格產生不利影響。
如果我們的股價波動,您可能會損失很大一部分投資。
我們普通股的市場價格可能會受到廣泛波動的影響,以應對本文件中描述的風險因素和其他我們無法控制的因素,例如投資者認爲與我們相當的公司估值的波動。此外,股票市場經歷了價格和成交量的波動,這些波動已經並將繼續影響許多公司的股權證券的市場價格。這些波動往往與這些公司的經營業績無關或不成比例。這些廣泛的市場和行業波動,以及一般的經濟、政治和市場狀況,如經濟衰退、利率變化或國際貨幣波動,可能會對我們普通股的市場價格產生負面影響。過去,許多經歷過股票市場價格波動的公司都會受到證券集體訴訟的影響。我們未來可能會成爲這類訴訟的目標。針對我們的證券訴訟可能導致巨額成本,並將我們管理層的注意力從其他業務上轉移,這可能會嚴重損害我們的業務。
賣空者使用的技術未來可能會壓低我們普通股的市場價格。
賣空是指出售賣家並不擁有的證券,而是從第三方借入的證券,目的是在以後回購相同的證券,然後返還給貸款人。賣空者希望從出售借入的證券和購買置換股票之間的證券價值下降中獲利,因爲賣空者預計在購買時支付的價格低於在出售中收到的價格。由於股票價格下跌符合賣空者的最佳利益,許多賣空者發佈或安排發佈對相關發行人及其業務前景的負面評論,以製造負面市場勢頭,並在賣空股票後爲自己創造利潤。這些做空攻擊導致了股票在市場上的拋售。擁有有限交易量的普通股和/或容易受到相對較高波動性水平影響的發行人,可能特別容易受到此類賣空者的攻擊。未來發表任何關於我們的此類文章可能會導致我們普通股的市場價格暫時或可能長期下降。如果我們繼續成爲不利指控的對象,我們可能不得不花費大量資源來調查這些指控和/或爲自己辯護。雖然我們會強烈防禦任何此類賣空者攻擊,但我們可能會受到適用的州法律或商業保密問題的限制,無法對相關賣空者採取行動。這種情況可能既昂貴又耗時,並可能分散我們管理團隊的注意力。
如果證券或行業分析師不發佈有關我們的研究或報告,或者如果他們對我們普通股的建議做出不利改變,那麼我們的股價和交易量可能會下降。
我們普通股的交易市場受到行業或證券分析師發佈的有關我們、我們的行業和市場的研究和報告的影響。如果沒有分析師選擇報道我們併發布有關我們的研究或報告,我們普通股的市場可能會受到嚴重限制,我們的股價可能會受到不利影響。作爲一家最近根據A法規完成首次公開募股(「IPO」)的小型公司,我們比大型競爭對手更有可能缺乏證券分析師的報道。此外,即使我們收到分析師的報道,如果一名或多名分析師停止報道我們或未能定期發佈有關我們的報告,我們可能會失去在金融市場的可見性,這反過來可能會導致我們的股價或交易量下降。如果一名或多名選擇報道我們的分析師發佈負面報告或不利地改變他們對我們普通股的建議,我們的股價可能會下跌。
作爲 “新興成長型公司” 根據《快速啓動我們的業務初創法案》(JOBS法案),我們被允許並且打算依賴某些披露要求的豁免。
根據《就業法案》,作爲一家「新興成長型公司」,我們被允許並打算依賴於某些披露要求的豁免。我們是一家新興的成長型公司,直到:
|
● |
財年的最後一天,我們的年總收入達到1235億美元或以上; |
|
● |
根據《證券法》下的有效登記聲明首次出售我們的普通股之日五週年後的財年最後一天; |
|
● |
在過去3年內,我們發行了超過10億美元的不可轉換債券的日期;或 |
|
● |
我們被視爲聯邦證券法定義的「大型加速發行人」的日期。 |
只要我們仍然是一家新興成長型公司,我們就不會被要求:
|
● |
根據2002年的《薩班斯-奧克斯利法案》,有關於我們財務報告的內部控制的審計報告; |
|
● |
遵守上市公司會計監督委員會可能通過的有關強制審計事務所輪換或提供有關審計和財務報表額外信息的審計報告補充的任何要求(核數師討論和分析); |
|
● |
根據「頻率發言權」和「薪酬發言權」條款,將某些高管薪酬事宜提交股東諮詢投票(要求不具約束力的股東投票批准某些高管的薪酬)和「金降落傘發言權」條款(要求不具約束力的股東投票批准與合併和某些其他業務合併有關的某些高管的金降落傘安排)2010年《多德-弗蘭克華爾街改革和消費者保護法案》; |
|
● |
在我們根據修訂後的1934年證券交易法提交的文件中包括詳細的薪酬討論和分析,並且可以降低有關高管薪酬的披露水平; |
|
● |
只能提供兩年的經審計財務報表和兩年的相關管理層對財務狀況和經營結果的討論和分析,或MD & A;以及 |
|
● |
根據《JOBS法案》第107條,有資格申請更長的分階段採用新的或修訂的財務會計準則。 |
我們打算利用所有這些減少的報告要求和豁免。
|
● |
由於根據委員會規則,我們也有資格成爲「小型報告公司」,因此我們已經可以使用這些減少的報告要求和豁免。例如,小型報告公司不需要獲得有關管理層對財務報告內部控制評估的核數師認證和報告;不需要提供薪酬討論和分析;不需要提供績效薪酬圖表或首席執行官薪酬比率披露;並且只能提供兩年的經審計財務報表和相關MD & A披露。 |
我們無法預測投資者是否會因爲我們依賴這些豁免而發現我們的證券的吸引力下降。如果投資者發現我們的普通股因我們的當選而失去吸引力,我們可能很難在未來的任何發行中籌集所有收益。
我們未能滿足納斯達克的持續上市要求可能會導致我們的普通股退市。
如果我們未能滿足納斯達克的持續上市要求,例如最低收盤出價要求,納斯達克可能會採取措施將我們的普通股退市。此類退市可能會對我們普通股的價格產生負面影響,並會損害您在您希望出售或購買我們普通股的能力。如果退市,我們將採取行動試圖恢復對納斯達克市場規則的遵守,但我們的普通股可能不會再次上市,此類行動可能無法穩定市場價格或改善我們普通股的流動性,防止我們的普通股跌破納斯達克最低買入價格要求或防止未來不遵守納斯達克市場規則。
我們的註冊證書包括論壇選擇條款,這可能會導致原告在針對我們的任何訴訟中獲得不利的結果。
我們的公司註冊證書包括一項法院選擇條款,要求股東對我們提出的任何非聯邦證券法規定的索賠都必須向特拉華州大法官州法院提起。該論壇選擇條款可能會限制投資者在他們認爲有利於此類爭議的司法論壇上提出索賠的能力,並可能會阻止就此類索賠提起訴訟。此外,該法院選擇條款可能會對股東在提出上述索賠時施加額外的訴訟費用,特別是如果股東不居住在特拉華州或特拉華州附近。
成爲上市公司的要求可能會給我們的資源帶來壓力,轉移管理’關注並影響我們吸引和留住合格董事會成員的能力。
作爲一家上市公司,我們招致了會計、法律和其他費用,這是我們作爲私人公司沒有招致的。我們產生了與我們的上市公司報告要求相關的成本。我們還產生與公司治理要求相關的成本,包括2002年薩班斯-奧克斯利法案下的要求,以及美國證券交易委員會(「美國證券交易委員會」)和納斯達克實施的規則和條例。我們預計這些規則和法規將增加我們的法律和財務合規成本,並使一些活動更加耗時和昂貴。此外,這些規則和條例可能會使我們更難或更昂貴地獲得某些類型的保險,包括董事和高級人員責任保險,我們可能會被迫接受降低的保單限額和承保範圍,或者爲獲得相同或類似的保險而產生更高的費用。這些要求的影響也可能使我們更難吸引和留住合格的人員加入我們的董事會、董事會委員會或擔任高管。我們目前正在評估和監測與這些規則和條例有關的事態發展,我們不能預測或估計我們可能產生的額外費用數額或這些費用的時間。
我們面臨證券集體訴訟的風險可能會增加。
從歷史上看,證券集體訴訟通常是在公司證券市場價格下跌後對其提起的。這種風險對我們來說尤其重要,因爲生物技術公司近年來經歷了大幅的股價波動。如果我們被起訴,可能會導致巨額成本並轉移管理層的注意力和資源,這可能會損害我們的業務。
有關前瞻性陳述的警示說明
本招股說明書包含某些涉及重大風險和不確定性的前瞻性陳述。除歷史事實陳述外,本招股說明書中包含的所有陳述均爲前瞻性陳述,包括有關我們的戰略、未來運營、未來財務狀況、未來收入、預計成本、前景、計劃、管理目標和預期市場增長的陳述。這些陳述涉及已知和未知的風險、不確定性和其他重要因素,可能導致我們的實際結果、績效或成就與前瞻性陳述中表達或暗示的任何未來結果、績效或成就存在重大差異。
「預期」、「相信」、「估計」、「預期」、「打算」、「可能」、「計劃」、「預測」、「項目」、「目標」、「潛在」、「將」、「將」、「可能」、「應該」、「繼續」以及類似的表述和類似表述旨在識別前瞻性表述,儘管並不是所有前瞻性表述都包含這些識別詞語。這些前瞻性陳述包括,除其他外,關於以下方面的陳述:
|
• |
我們在短期內繼續經營的能力取決於我們能否成功籌集額外的股權或債務融資來爲我們的運營提供資金; |
|
• |
我們未來臨床試驗的成功; |
|
• |
我們目前沒有產品銷售收入來源; |
|
• |
來自現有產品或可能出現的新產品的競爭; |
|
• |
如果我們未能遵守美國和外國監管要求,監管機構可能會限制或撤回我們可能獲得的任何營銷或商業化批准,並對我們處以可能對我們業務造成重大損害的其他處罰; |
|
• |
我們可能無法獲得美國或外國監管機構的批准,因此無法將我們的候選產品商業化; |
|
• |
針對我們的業務、技術和候選產品,實施我們的業務模式和戰略計劃; |
|
• |
潛在的產品責任索賠; |
|
• |
我們依賴第三方供應和製造合作伙伴爲我們的研發、臨床前和臨床試驗設備提供材料和部件並製造這些設備; |
|
• |
我們建立或維持合作、許可或其他安排並保留受合作影響的候選產品的商業權利的能力; |
|
• |
我們和第三方保護知識產權的能力以及我們在不侵犯他人知識產權的情況下運營業務的能力; |
|
• |
我們充分支持未來增長的能力; |
|
• |
我們對支出、持續虧損、未來收入和資本需求的估計; |
|
• |
我們有能力吸引和留住關鍵管理人員和技術人員以有效管理我們的業務; |
|
• |
與我們識別財務報告控制中的重大弱點相關的風險; |
|
• |
我們使用從任何後續私募或公共融資中收到的淨收益; |
|
• |
影響我們、我們的主要製造商或供應商的自然災害; |
|
• |
我們與醫療保健專業人員和組織建立關係的能力; |
|
• |
總體經濟不確定性,對醫療程序支出產生不利影響; |
|
• |
我們股票市場價格的波動;和 |
|
• |
發行股權獎勵對當前股東的潛在稀釋。 |
這些前瞻性陳述只是預測,我們可能實際上無法實現前瞻性陳述中披露的計劃、意圖或預期,因此您不應過度依賴我們的前瞻性陳述。實際結果或事件可能與我們在前瞻性陳述中披露的計劃、意圖和預期存在重大差異。我們的這些前瞻性陳述主要基於我們對未來事件和趨勢的當前預期和預測,我們認爲這些事件和趨勢可能影響我們的業務、財務狀況和經營業績。我們在本招股說明書中包含的警告性陳述中納入了重要因素,這些因素可能導致實際未來結果或事件與我們做出的前瞻性陳述存在重大差異。我們的前瞻性陳述並不反映我們未來可能進行的任何收購、合併、處置、合資企業或投資的潛在影響。
閣下在閱讀本招股說明書時,應了解我們的實際未來業績可能與我們的預期有重大差異。我們不承擔任何義務更新任何前瞻性陳述,無論是由於新信息、未來事件或其他原因,除非適用法律要求。
收益的使用
假設我們根據本招股說明書完成最高發行,扣除承銷折扣和佣金以及我們應付的估計發行費用,假設承銷商的超額配股選擇權沒有行使,我們估計發行的淨收益將約爲900萬美元。這些估計不包括本次發行中出售的普通股和預融資股(如果有)的行使收益(如果有)。行使預融資令後,我們將收到名義收益(如有)。
我們打算將淨收益用於(i)我們的臨床試驗;(ii)其他研究和開發;(iii)知識產權開發和(iv)流動資金。
即使我們完成了以下提供,我們也將需要大量額外融資來完成我們的臨床試驗並商業化我們的候選產品。我們沒有承諾此類額外所需融資,並且可能會被要求通過出售額外股權證券來籌集此類融資,這些證券的價格可能低於本次發行中我們普通股的發行價。
截至本招股說明書的日期,我們不能確切地說明本次發行爲我們帶來的淨收益的所有特定用途。因此,我們的管理層將在運用這些收益方面擁有廣泛的酌處權。未立即用於上述概述用途的淨髮售收益將投資於短期投資,如貨幣市場基金、商業票據、美國國庫券和類似的證券投資,等待其使用。
股利政策
迄今爲止,我們尚未對普通股支付任何股息,並且預計在可預見的未來也不會支付任何股息。普通股股息的宣佈和支付由我們的董事會自行決定,並將取決於(除其他外)我們的經營業績、財務狀況、資本要求、合同限制或董事會可能認爲相關的其他因素。我們目前預計使用所有可用資金爲我們業務的未來發展和擴張提供資金,並且預計在可預見的未來不會向我們的普通股支付股息。
稀釋
如果您在本次發行中投資我們的證券,您的權益將立即被稀釋,其幅度爲本次發行中出售的普通股單位或PFW單位的購買者支付的公開發行價格與本次發行後每股普通股調整後的有形淨現值之間的差額。
截至2024年6月30日,我們的有形淨資產爲530萬美元,即每股普通股5.50美元。每股有形淨資產代表我們的有形資產總額,減去我們的總負債,再除以我們普通股的流通股數。
截至2024年6月30日,我們的預計有形淨賬面價值爲530萬美元,即每股普通股4.59美元。每股預計有形淨價值生效:(i)於2024年7月發行與RTI許可協議相關的12,500股普通股;和(ii)在2024年6月30日之後以無現金方式行使期權後發行177,246股普通股。
攤薄是指購買者在此次發售中支付的每單位金額與發售後調整後普通股每股有形賬面淨值的預計值之間的差額。在實施上述備考調整及在本次發售中以每單位14.31美元的假設發行價出售698,812股普通股後,扣除承銷折扣和佣金及估計應支付的發售開支,扣除承銷折扣及佣金及估計應支付的發售開支,但剔除根據本次發售而發行的普通權證及預先出資認股權證的行使所得款項(如有),以及未對2024年6月30日之後我們的有形賬面淨值任何其他變化作出調整,我們的備考有形賬面淨值將爲每股7.71美元。這意味着我們的現有股東在預計基礎上的每股有形賬面淨值立即增加3.12美元,對以建議的公開發行價購買證券的新投資者的每股立即稀釋6.60美元。下表說明了截至2024年6月30日對新投資者的每股有形賬面淨值稀釋情況:
|
假定的單位公開發行價 |
$ | 14.31 | ||||||
|
截至2024年6月30日的歷史每股有形淨值 |
$ | 5.50 | ||||||
|
截至2024年6月30日的預計每股有形賬面淨值 |
$ | 4.59 | ||||||
|
本次發行可歸因於現有股東的每股有形淨賬面價值預計增加。 |
$ | 3.12 | ||||||
|
預計爲本次發行後調整後的每股有形賬面淨值 |
$ | 7.71 | ||||||
|
對新投資者每股有形賬面淨值的稀釋 |
$ | 6.60 |
假設公開招股價格每股14.31美元每增加(減少)1.00美元,在扣除估計的承銷折扣和佣金以及估計應支付的發售費用後,我們向現有投資者提供的經調整的每股有形賬面淨值將增加(減少)0.35美元,並將在本次發行中向新投資者增加(減少)每股攤薄0.65美元。我們也可以增加或減少此次發行的證券數量。假設假設公開發售價格保持不變,在扣除承銷折扣和佣金及估計應支付的發售費用後,吾等每增加(減少) 100,000股股份將增加(減少)吾等的經調整每股有形賬面淨值0.71美元,以及向購買本次發售證券的新投資者攤薄每股0.71美元。上述資料僅供參考,並將根據吾等與承銷商在定價時厘定的實際公開發售價格及本次發售的其他條款作出調整。
The number of shares of common stock to be outstanding after this offering is based on 962,374 shares outstanding as of June 30, 2024 and excludes:
|
● |
292,234 shares of common stock underlying outstanding warrants at a weighted average exercise price of $3.30 per share; |
|
● |
216,483 shares of common stock underlying outstanding options with a weighted average exercise price of $36.43 per share; |
|
● |
33,250 shares of common stock underlying outstanding convertible notes at a weighted average conversion price of $40.00 per share; |
|
● |
75,633 shares available for future issuance under the Autonomix Medical, Inc. 2023 Stock Plan; |
| ● |
698,812 shares of common stock underlying the Common Warrants issuable in this offering; and |
|
● |
41,928 shares of common stock underlying the Representative Warrants issuable to the underwriter in connection with this offering. |
We may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
CAPITALIZATION
The following table sets forth our cash and cash equivalents and capitalization as of June 30, 2024:
|
• |
on an actual basis; |
|
• |
on a pro forma basis to give effect to (i) the issuance of 12,500 shares of common stock in connection with the RFI license agreement in July 2024; and (ii) the issuance of 177,246 shares of common stock upon the exercise of warrants on a cashless basis subsequent to June 30, 2024; |
|
• |
on a pro forma adjusted basis to give further effect to (i) the issuance and sale of 698,812 Units in this offering at an assumed offering price of $14.31 per Unit, which was the closing price of our common stock as reported on Nasdaq on October 31, 2024, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. |
Our capitalization following the closing of this offering will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. You should read this table in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes appearing elsewhere in this prospectus.
|
Adjusted |
||||||||||||
|
Actual |
Pro Forma |
Pro Forma |
||||||||||
|
(in thousands, except par value and share data) |
||||||||||||
|
Cash and cash equivalents |
$ | 6,751 | $ | 6,751 | $ | 15,731 | ||||||
|
Notes Payable |
$ | 1,043 | $ | 1,043 | $ | 1,043 | ||||||
|
Stockholders’ equity: |
||||||||||||
|
Common stock, par value $0.001 per share: 500,000,000 shares authorized as of June 30, 2024; 962,374 shares issued and outstanding as of June 30, 2024; 1,152,120 shares issued and outstanding pro forma; and 1,850,932 shares issued and outstanding adjusted pro forma |
$ | 1 | $ | 1 | $ | 2 | ||||||
|
Additional paid-in capital |
$ | 46,956 | $ | 47,056 | $ | 56,036 | ||||||
|
Accumulated deficit |
$ | (41,668 | ) | $ | (41,768 | ) | $ | (41,768) | ||||
|
Total stockholders’ equity |
$ | 5,289 | $ | 5,289 | $ | 14,269 | ||||||
|
Total capitalization |
$ | 6,332 | $ | 6,332 | $ | 15,312 | ||||||
|
(1) |
An $1.00 increase or decrease in the assumed public offering price of $14.31 per Unit, which was the closing price of our common stock as reported on NASDAQ on October 31, 2024, would increase or decrease, respectively, our pro forma as adjusted cash and cash equivalents, additional paid-in capital, total stockholders’ equity, and total capitalization by approximately $642,907 assuming the number of securities offered by us, as set forth on the cover page of this prospectus, remains the same, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of securities to be issued in this offering. An increase or decrease of 100,000 in the number of Units offered by us would increase or decrease, respectively, our pro forma as adjusted cash and cash equivalents, additional paid-in capital, total stockholders’ equity, and total capitalization by $1,316,520 assuming that the assumed public offering price remains the same, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. The information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering as determined between us and the underwriters. |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND
RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with the financial statements and the related notes appearing elsewhere in this prospectus. This discussion contains forward-looking statements reflecting our current expectations that involve risks and uncertainties, including those set forth under "Cautionary Statement About Forward-Looking Statements." Actual results and the timing of events could differ materially from those discussed and other expectations expressed in our forward-looking statements as a result of many factors, including but not limited to those discussed herein and in the "Risk Factors” section above.
Overview
We are a development stage medical device development company focused on advancing innovative technologies for sensing and treating disorders relating to the nervous system. Our first-in-class technology platform includes a catheter-based microchip-enabled sensing array that can detect and differentiate neural signals with a high degree of sensitivity as demonstrated in animal studies. We calculate sensitivity in units of minimum signal detection voltage in micro volts (uV) time area of the electrode (square millimeters). It is a combined measure that is related to the signal resolving power and spatial resolution of the system. For the BSC Orion, the nearest device on the market, the metrics are 10uV for signal detection levels, and roughly 0.4mm by 0.5mm for the electrode dimensions. For the Autonomix device, the metrics are <1uV for signal detection levels and roughly 0.02mm by 0.03mm for the electrode dimensions. The differences in these metrics result in a calculation of 3,000 times greater sensitivity for the Autonomix device. We believe, if we can recreate these results in clinical trials, this will enable a method of transvascular targeting, treating, and confirming treatment of diseases involving the nervous system throughout the body that is not currently available and may be capable of filling a wide range of unmet medical needs.
We are initially developing our technology for patients with pancreatic cancer, a condition that can cause debilitating pain and need a more effective solution. However, we believe our technology constitutes a platform with the potential to address dozens of indications in a range of areas including chronic pain management from all causes, hypertension, cardiovascular disease and a wide range of other nerve-related disorders.
Our development efforts can be divided into to two sub parts: sensing and treatment, where sensing is focused on identifying neuronal activity that may be associated with a disorder with enough precision to enable targeted therapy with ablation. While the treatment may vary depending on the disorder, in our initial indications this will involve energy-based ablation (deliberate tissue damage, also referred to as denervation) intended to stop unwanted neuronal activity.
Our sensing catheter has already been developed sufficiently to demonstrate in animal models successful identification of a signal from a specific nerve before ablation and confirmation of termination of the signal from the treated nerve after ablation. We are now in the process of improving the assembly of this catheter to meet the standards required for human use and developing an RF ablation catheter designed specifically for treatment in the vessels of the pancreatic region. In parallel with this effort, we are currently conducting a first-in-human demonstration of transvascular ablation (without the use of our sensing technology) to relieve pain associated with pancreatic cancer. Once these two efforts are completed, we plan to bring sensing and treatment together in a pivotal clinical trial to enable the regulatory clearance and commercial launch of our technology. As stated above, we are a development stage company and there is no guarantee that the results of any trials will produce positive results or that the results will support our claims.
Recent Developments
On July 10, 2024, we entered into a license agreement (the “Agreement”) with RF Innovations, Inc. (“RFI”), a privately held medical technology company, to license products utilizing RFI’s intellectual property related to its Apex 6 Radiofrequency Generator (the “Licensed Products”). The Apex 6 Generator is a United States Food and Drug Administration (“FDA”) cleared ablation technology designed to lesion neural tissue for pain management in the peripheral nervous system. Pursuant to the Agreement, RFI granted us a perpetual non-exclusive worldwide royalty free fully paid license related to the Licensed Products, provided that the license did not include the right to sell certain products to customers for the treatment of spine pain. In connection with the Agreement, we issued RFI 12,500 unregistered shares of our common stock as consideration for the license. The Agreement provides RFI the right to terminate the license if we breach any representation, warranty or covenant contained in the Agreement, subject to any relevant cure periods, or if we are subject to a bankruptcy or insolvency event.
On January 26, 2024, we consummated our IPO. In the IPO, we sold a total of 111,962 shares of common stock at a purchase price of $100.00 per share for gross proceeds of $11.2 million and net proceeds of $9.8 million. On May 13, 2024, we cancelled 53 shares represented in the IPO for payment disputes. In connection with the closing of the IPO, a portion of our convertible notes were converted into 16,750 shares of our common stock. Upon the closing of the IPO, certain notes were to be automatically converted according to their terms into our common stock to the extent and provided that certain holders of these notes are not permitted to convert such notes to the extent that the holders or any of its affiliates would beneficially own in excess of 4.99% of our common stock after such conversion. Due to this 4.99% limitation, principal representing $1.3 million, or 33,250 shares, of these notes remains outstanding.
On January 26, 2024, as part of our IPO, we issued a warrant to purchase 2,989 shares pursuant to the agreement with the selling agent in our IPO. These warrants equaled approximately 2.675% of the 111,962 shares sold in our IPO.
On January 29, 2024, we issued a warrant to purchase 80,000 shares (the “Warrant”) pursuant to the Termination Agreement noted in Note 6 – Related Party Transactions. The shares underlying the Warrant are subject to a lockup agreement for a period of six months after the closing of the IPO with respect to 12.5% of the shares issued and twelve months after the closing of the IPO for the remainder of the shares. In connection with the Termination Agreement, the Company agreed to register the resale of the shares of common stock underlying the Warrant.
Results of Operations for the Three Months Ended June 30, 2024 Compared to the Three Months Ended June 30, 2023
Below is a summary of the results of operations (in thousands):
|
Three Months Ended June 30, |
||||||||||||||||
|
Change |
Change |
|||||||||||||||
|
2024 |
2023 |
( $ ) |
( % ) |
|||||||||||||
|
Operating expenses: |
||||||||||||||||
|
General and administrative |
$ | 1,799 | $ | 503 | $ | 1,296 | 258 | % | ||||||||
|
Research and development |
954 | 368 | 586 | 159 | % | |||||||||||
|
Total operating expenses |
$ | 2,753 | $ | 871 | $ | 1,882 | 216 | % | ||||||||
General and Administrative Expense
General and administrative expense was $1.8 million for the three months ended June 30, 2024 compared to $0.5 million for the same period in 2023. This $1.3 million increase was driven primarily by increases in officer and employee compensation and benefits of $0.6 million, as we expanded our management team, stock-based compensation of $0.3 million, legal and professional fees of $0.2 million, insurance expense of $0.1 million, State of Delaware franchise tax of $0.1 million and other expenses of $0.1 million, offset by a decrease in advertising of $0.1 million
Research and Development Expense
Research and development expense was $1.0 million for the three months ended June 30, 2024 compared to $0.4 million for the same period in 2023. The increase in research and development expenses during the current year was mainly attributed to clinical trial planning and development cost. We expect to incur increased research and development costs in the future as we continue our clinical trial.
Interest expense
For the three months ended June 30, 2024, we had interest expense of less than $0.1 million, related to the amortization of debt discount. Interest expense was $0 during the three months ended June 30, 2023 as there was no comparable instrument or expense in the prior period.
Interest income
For the three months ended June 30, 2024, we had interest income of $0.1 million. Interest income for the three months ended June 30, 2023 was less than $0.1 million due to relatively lower cash balances.
Results of Operations for the Year Ended March 31, 2024 Compared to the Year Ended March 31, 2023
Below is a summary of the results of operations (in thousands):
|
Year Ended March 31, |
||||||||||||||||
|
Change |
Change |
|||||||||||||||
|
2024 |
2023 |
( $ ) |
( % ) |
|||||||||||||
|
Operating expenses: |
||||||||||||||||
|
General and administrative |
$ | 5,249 | $ | 1,245 | $ | 4,004 | 322 | % | ||||||||
|
Research and development |
2,225 | 745 | 1,480 | 199 | % | |||||||||||
|
Warrant expense - termination agreement |
4,556 | — | 4,556 | — | ||||||||||||
|
Total operating expenses |
$ | 12,030 | $ | 1,990 | $ | 10,040 | 505 | % | ||||||||
General and Administrative (G&A). G&A expenses increased by $4.0 million compared to the same period in 2023, primarily due to increases in advertising of $1.7 million related to our IPO, officer and employee compensation and benefits of $0.7 million, stock-based compensation of $0.6 million, professional fees of $0.6 million, legal fees of $0.2 million, insurance expense of $0.1 million and travel expense of $0.1 million.
Research and Development (R&D). R&D expenses increased by $1.5 million compared to the same period in 2023, primarily due to clinical trial execution and product development cost. We expect to incur increased research and development costs in the future as we continue our clinical trial and product development efforts.
Warrant expense – termination agreement
We had warrant expense of $4.6 million related to a license termination agreement. See Note 2 - Warrant Liability and Fair Value of Financial Instruments to the financial statements for additional information. Warrant Expense – termination agreement was $0 during the same period in 2023 as there was no comparable instrument.
Warrant liability mark-to-market
We had expense for mark-to-market adjustments of warrants of $3.4 million. Warrant Liability - mark-to market adjustment was $0 during the same period in 2023 as there was no comparable instrument.
Interest expense
We had interest expense of less than $0.1 million, related to the amortization of debt discounts. Interest expense was $0 during the same period in 2023 as there was no comparable instrument.
Interest income
We had interest income of $0.1 million. Interest income for the same period in 2023 was $0.
Liquidity and Capital Resources
On June 30 2024, we had cash of $6.8 million and working capital of $6.3 million. We have historically funded our operations from proceeds from debt and equity sales. In June 2023, we completed a financing with several accredited investors for the sale of 71,001 shares of common stock with gross proceeds of $2.8 million. Additionally, the Company received proceeds of $2.0 million in unsecured, non-interest bearing convertible promissory notes (the “Notes”) and accompanying warrants (the “Bridge Financing Warrants”) (collectively, the “Bridge Offering”) that will mature on December 31, 2025. On January 26, 2024, we completed our IPO of common stock. In the IPO, we sold a total of 111,962 shares of common stock at a purchase price of $100.00 per share for gross proceeds of $11.2 million and net proceeds of $9.8 million. On May 13, 2024, we cancelled 53 shares represented in the IPO for payment disputes. We estimate our current cash resources are sufficient to fund our operations into but not beyond the second calendar quarter of 2025.
Our plan of operations is primarily focused on developing our product candidate, with the product candidate in the proof-of-concept stage at this time. We are initially focusing on the treatment of pain associated with pancreatic cancer and we have designed our commercialization efforts around this as our first proposed indication for use.
We will need to raise additional capital to meet our obligations and execute our business plan. We estimate that we will require additional financing of approximately $40 million to fund our operations through clinical phase. The timing and costs of clinical trials are difficult to predict and trial plans may change in response to evolving circumstances and as such the foregoing estimates may prove to be inaccurate. If we are unable to raise sufficient funds, we will be required to develop and implement an alternative plan to further extend payables, reduce overhead or scale back our business plan until sufficient additional capital is raised to support further operations. There can be no assurance that such a plan will be successful. The Company recognizes it will need to raise additional capital to continue to execute its business plan, including obtaining regulatory clearance for its products currently under development and commercializing and generating revenues from products under development. There is no assurance that additional financing will be available when needed or that management will be able to obtain financing on terms acceptable to the Company. A failure to raise sufficient capital, generate sufficient product revenues, control expenditures and regulatory matters, among other factors, will adversely impact the Company’s ability to meet its financial obligations as they become due and payable and to achieve its intended business objectives. If the Company is unable to raise sufficient additional funds, it will have to scale back its operations.
Summary of Cash Flows for the Three Months Ended June 30, 2024 and 2023
Cash used in operating activities
Net cash used in operating activities was $1.9 million during the three months ended June 30, 2024, consisting of a net loss of $2.7 million and a decrease in operating assets and liabilities of $0.5 million. The change in operating assets and liabilities included sources of cash from a decrease in other current assets of $0.5 million and an increase in accrued expenses of $0.2 million offset by a use of cash decrease for accounts payable of $0.2 million. The decrease in other current assets was driven primarily by the receipt of funds from our marketing partner that were a holdback from our IPO and the amortization of prepaid insurance costs. The increase in accrued expenses and the decrease in accounts payable are offsetting and are driven primarily by the timing of receipt of vendor invoices. Non-cash items consisted of stock-based compensation of $0.4 million.
Cash used in investing activities
Net cash used in investing activities was $5 thousand for the three months ended June 30, 2024 related to the purchase of computer hardware and software.
Cash provided by financing activities
Net cash provided by financing activities was zero for the three months ended June 30, 2024.
Net cash provided by financing activities was $2.7 million for the three months ended June 30, 2023 consisting of $2.8 million from the sale of common stock. We also paid $0.1 million in offering costs related to our IPO.
Summary of Cash Flows for the Years Ended March 31, 2024 and 2023
Cash used in operating activities
Net cash used in operating activities was $6.6 million during the year ended March 31, 2024, consisting of a net loss of $15.4 million and a change in operating assets and liabilities of $0.1 million. The change in operating assets and liabilities included sources of cash from an increase in accounts payable of $0.3 million and accrued expenses of $0.3 million offset by a use of cash for other current assets of $0.5 million. The increases in accounts payable and accrued expenses were driven primarily by increased research and development costs for the development of our medical devices, general and administrative costs consisting of professional fees, officer compensation and legal expenses. The increase in other current assets was driven primarily by prepaid insurance costs. Non-cash items consisted of $4.6 million for warrant expense – termination agreement, $3.4 million for warrant liability – mark-to-market adjustment, stock-based compensation of $0.6 million and depreciation and amortization of $0.1 million.
Net cash used in operating activities was $1.9 million during the year ended March 31, 2023, consisting of a net loss of $2.0 million and an increase in operating assets and liabilities of $0.1 million, which primarily consisted of an increase in accounts payable.
Cash used in investing activities
Net cash used in investing activities was $19 thousand for the year ended March 31, 2024, related to the purchase of computer hardware and software.
Net cash used in investing activities was $0 for the year ended March 31, 2023.
Cash provided by financing activities
Net cash provided by financing activities was $14.4 million for the year ended March 31, 2024 consisting of $10.9 million of gross proceeds from the sale of common stock related to our IPO, $2.8 million from the sale of common stock and $2.0 million of cash proceeds from convertible notes. We also paid $1.3 million in issuance costs related to our IPO.
Net cash provided by financing activities was $0.7 million for the year ended March 31, 2023, comprised of $0.7 million from the sale of common stock.
Contractual Obligations and Commitments
None.
Employment Arrangements
We have agreements with key employees to provide certain benefits, including salary and other wage-related benefits, in the event of termination. In addition, we have adopted a severance policy for certain key members of executive management to provide certain benefits, including salary and other wage-related benefits, in the event of termination without cause. In total, these benefits would amount to a range of $1.1 million to $1.6 million using the rate of compensation in effect at June 30, 2024.
Off-balance Sheet Arrangements
As of June 30, 2024 and March 31, 2024, we did not have any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities, established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes.
Critical Accounting Estimates
The financial statements in this prospectus have been prepared in accordance with generally accepted accounting principles in the United States of America (“GAAP”). The preparation of financial statements in conformity with GAAP requires management to make estimates, assumptions and judgments that affect the amounts reported in the financial statements, including the notes thereto. We consider critical accounting policies to be those that require more significant judgments and estimates in the preparation of our financial statements, including the following: research and development expenses, warrants, and stock-based compensation. Management relies on historical experience and other assumptions believed to be reasonable in making its judgments and estimates. Actual results could differ materially from those estimates.
Management believes its application of accounting policies, and the estimates inherently required therein, are reasonable. These accounting policies and estimates are periodically reevaluated, and adjustments are made when facts and circumstances dictate a change.
Our accounting policies are more fully described under the heading “Description of the Business, Basis of Presentation and Summary of Significant Accounting Policies” in Note 1 to our Financial Statements included in this prospectus.
We believe that the following accounting policies are the most critical to aid in fully understanding and evaluating our reported financial results, and they require our most difficult, subjective or complex judgments, resulting from the need to make estimates about the effect of matters that are inherently uncertain.
Components of our Results of Operations and Financial Condition
Operating expenses
We classify our operating expenses into three categories: (i) research and development, (ii) general and administrative and (iii) warrant expense – termination agreement.
Research and development.
Research and development expenses consist primarily of:
|
• |
costs incurred to conduct research, such as animal research; |
|
|
• |
costs related to the design and development of our technology, including fees paid to contract engineering firms and contract manufacturers; |
|
|
• |
salaries and expenses, including stock-based compensation, related to our employees primarily engaged in research and development activities; |
|
|
• |
fees paid to clinical consultants, clinical trial sites and vendors, including clinical research organizations, in preparation for clinical trials and our applications with the FDA; |
|
|
• |
costs to develop our intellectual property; and |
|
|
• |
costs related to compliance with regulatory requirements. |
We expect our research and development expenses to increase in the future as we advance our product into and through clinical trials, pursue additional regulatory approvals of our product in the United States, and continue commercial development of our device(s). The process of conducting the necessary clinical research to obtain regulatory approval is costly and time-consuming. The probability of success for our technology may be affected by a variety of factors including: the quality of our product, early clinical data, investment in our clinical program, competition, manufacturing capability and commercial viability. We may not succeed in achieving all necessary regulatory approvals for our product candidates. As a result of the uncertainties discussed above, we are unable to determine the duration and completion costs of our research and development process or when and to what extent, if any, we will generate revenue from the commercialization and sale of our device.
General and administrative
General and administrative expenses consist of personnel related costs, which include salaries, as well as the costs of professional services, such as accounting and legal, facilities, information technology, stock-based compensation for general and administrative personnel, insurance, travel costs and other administrative expenses and costs to defend our patents. We expect our general and administrative expenses to increase due to the IPO, the anticipated growth of our business and related infrastructure, as well as accounting, insurance, investor relations and other costs associated with being a public company.
Advertising
It is our policy to expense advertising costs as incurred. Advertising expenses are included within general and administrative expenses within the statement of operations. For the three months ended June 30, 2024 and 2023, the Company recorded less than $0.1 million and $0.1 million, respectively. For the years ended March 31, 2024 and 2023, the Company recorded $1.7 million and $0.1 million, respectively.
Stock-based compensation
Stock-based compensation transactions are recognized as compensation expense in the statements of operations based on their fair values on the date of the grant. The expense for equity awards expected to vest is recognized over the applicable vesting period of the stock award using either the straight-line method or the accelerated method, depending on the vesting structure, and is included in general and administrative. We estimate the fair value of options granted using the Black-Scholes option pricing model. This estimate uses assumptions regarding a number of inputs that require us to make significant estimates and judgments. The expected volatility assumption was based on industry peer information.
Accounting for Warrants
We issued warrants to purchase shares of common stock (i) in connection with the Bridge Offering, (ii) as part of selling agent compensation in 2024, and (iii) in connection with the Exclusive License Termination Agreement (the “Termination Agreement”). We accounted for such warrants in accordance with Accounting Standards Codification (“ASC”) Topic 480-10, Distinguishing Liabilities from Equity and ASC Topic 815-40, Derivatives and Hedging – Contracts in Entity’s Own Equity. Based on this guidance, we determined that warrants issued in connection with the Termination Agreement should be accounted for as a liability and the remaining warrants issued meet the requirements for equity classification. Liability classified warrants are subject to remeasurement at each balance sheet date, while equity classified warrants are valued at inception only.
Bridge Financing Warrants
The fair value of the Bridge Financing Warrants is estimated using a Monte Carlo simulation model with probability-weighted expected return method ("PWERM") based on the probabilities of different potential outcomes for the Notes issued with the Bridge Financing Warrants. The outcomes considered included (i) qualified financing as part of our planned IPO at various points in time and (ii) repayment in cash at maturity. Any increase in the amount of time expected until a qualified financing event and/or a reduction in the likelihood of a qualified financing event occurring during the term of the Notes would likely increase the fair value of the warrant, while the inverse of each scenario would have the opposite effect. The significant judgments and assumptions to the Monte Carlo simulation include the Company’s stock price, volatility based on a selection of publicly held peer companies, discount rate, and a discount for lack of marketability.
Common Stock Fair Value – The fair value of our common stock price was determined through a back solve, solving for the stock price that results in the average total value of the Notes and the warrants being equal to the cash proceeds received in the transaction it was issued at across one million iterations of the simulation.
Historical Volatility – We determine the expected volatility by weighing the historical average volatilities of publicly traded industry peers. Our intention is to consistently apply this methodology using the same or similar public companies until a sufficient amount of historical information regarding the volatility of our common stock becomes available. We will monitor our peer group for circumstances that may require a change to the composition or make-up of the entities and will identify if/when more suitable companies whose stock prices are publicly available would be utilized in the calculation.
Discount Rate - The rate is chosen based on private equity rates of return as described in the AICPA Practice Aid on Valuation of Privately-Held-Company Equity securities Issued as Compensation, choosing the rate at the lower end of the range.
Credit Rating – Our credit rating impacts the identification and calculation of the discount rate.
Discount for lack of marketability – Subsequent to the IPO, any shares issued pursuant to an exercise of the Bridge Financing Warrants, would be subject to a six-month lock-up. Consistent with AICPA’s Accounting and Valuation Guide: Valuation of Privately-Held-Company Equity Securities Issued as Compensation, the Finnerty model was used to estimate the discount for lack of marketability.
The fair value of the Notes and Bridge Financing Warrants is calculated such that they will combine to equal the cash purchase price of the Bridge Offering. Any changes in these assumptions will impact how the transaction price from the Bridge Offering is distributed between the Notes and the Bridge Financing Warrants.
Termination Agreement Warrants
The fair value of the Termination Agreement Warrants is estimated using a discounted cash flow model under various scenarios and used the probability-weighted expected return method (“PWERM”) comparing the probabilities of different outcomes. The outcomes considered included (i) qualified financing as part of our planned IPO at various points in time and (ii) possibility of default whereby the investor receives nothing. Any increase in the amount of time expected until a qualified financing event and/or a reduction in the likelihood of a qualified financing event occurring during the term of the warrant would decrease the fair value of the warrant, while the inverse of each scenario would have the opposite effect.
Additional significant assumptions and judgments used in preparing the discounted cash flow model include:
Discount Rate - The rate is chosen based on private equity rates of return as described in the AICPA Practice Aid on Valuation of Privately-Held-Company Equity Securities Issued as Compensation, choosing the rate at the lower end of the range.
Credit Rating – Our credit rating impacts the identification and calculation of the discount rate.
Any ongoing improvements in our credit rating would have the effect of driving down the discount rate used in the periodic re-measurement of the Termination Agreement warrants. Reductions in the Company’s discount rate would increase the fair value of the Termination Agreement warrants, while an increase in this factor will have an opposite effect.
Other Warrants
The fair value of equity-based warrants issued is estimated using the Black-Scholes option pricing model. The significant judgments and assumptions used in applying the Black-Scholes option pricing model include the underlying common stock at the measurement dates, the expected term, expected dividend yield and historical volatility of comparable companies’ stock.
Common Stock Fair Value – Prior to our IPO, we periodically sold shares of our common stock for cash in an arms-length transaction. We consider these transactions as indicative of the fair value of our common stock when applying the Black-Scholes option pricing model. Subsequent to our IPO, we base the value of our shares on observable share data.
Expected Term – The estimate of the expected term of awards was determined in accordance with the contractual term of the arrangement.
Expected Dividend Yield – We have not declared or paid any cash dividends and do not presently intend to pay any in the foreseeable future. We have no plans or expectations that this assumption will change in the foreseeable future.
Historical Volatility – We determine the expected volatility by weighing the historical average volatilities of publicly traded industry peers. Our intention is to consistently apply this methodology using the same or similar public companies until a sufficient amount of historical information regarding the volatility of our common stock becomes available. We will monitor our peer group for circumstances that may require a change to the composition or make-up of the entities and will identify if/when more suitable companies whose stock prices are publicly available would be utilized in the calculation.
A decrease in volatility and expected term will decrease the estimated fair value of the warrant, while an increase in these factors will have an opposite effect.
BUSINESS
Overview
We are a development stage medical device development company focused on advancing innovative technologies for sensing and treating disorders relating to the nervous system. Our first-in-class technology platform includes a catheter-based microchip-enabled sensing array that can detect and differentiate neural signals with a high degree of sensitivity as demonstrated in animal studies. We are initially developing our technology for patients with pancreatic cancer, a condition that can cause debilitating pain and needs a more effective solution. However, we believe our technology constitutes a platform with the potential to address dozens of indications in a range of areas including chronic pain management from all causes, hypertension, cardiovascular disease and a wide range of other nerve-related disorders.
We calculate sensitivity in units of minimum signal detection voltage in micro volts (uV) time area of the electrode (square millimeters). It is a combined measure that is related to the signal resolving power and spatial resolution of the system. For the BSC Orion, the nearest device on the market, the metrics are 10uV for signal detection levels, and roughly 0.4mm by 0.5mm for the electrode dimensions. For the Autonomix device, the metrics are <1uV for signal detection levels and roughly 0.02mm by 0.03mm for the electrode dimensions. The differences in these metrics result in a calculation of 3,000 times greater sensitivity for the Autonomix device. We believe, if we can recreate these results in clinical trials, this will enable a method of transvascular targeting, treating, and confirming treatment of diseases involving the nervous system throughout the body that is not currently available and may be capable of filling a wide range of unmet medical needs.
Our development efforts can be divided into to two sub parts: diagnostic and therapeutic, where diagnostic is focused on sensing and identifying neuronal activity that may be associated with a disorder with enough precision to enable targeted therapy with ablation. Our sensing catheter has already been developed sufficiently to demonstrate in animal models successful identification of a signal from a specific nerve bundle before ablation and confirmation of termination of that signal from the treated nerves after ablation. We are now in the process of improving the design of this catheter to meet the standards required for human use. In parallel with this effort, we are conducting a first-in-human demonstration of transvascular ablation to relieve pain associated with pancreatic cancer, with the intent to bring sensing and treatment together in a future pivotal clinical trial to enable the commercial launch of our technology. We are a development stage company and there is no guarantee that the results of any trials will produce positive results or that the results will support our claims.
We believe one of the most demanding aspects of our commercialization plan will be scaling up from our existing sensing prototype to a robust commercial version. Today, our sensing device is hand built and includes a combination of hand-crafted and 3D printed parts. We have not yet assembled or tested what will be the commercial version of our proposed device. Even if our proposed device is cleared for commercial use, there is no assurance that we will be able to successfully build such device on a commercial scale.
As of June 30, 2024, we had an accumulated deficit of $41.7 million, negative cash flows from operating activities of $1.9 million and working capital of $6.3 million, which raises substantial doubt about our ability to continue as a going concern. Further, we have incurred and expect to continue to incur significant costs in pursuit of our business plans. We cannot assure you that we will be successful in raising additional funds. These factors, among others, raise substantial doubt about our ability to continue as a going concern.
Our Technology
Targeting the Peripheral Nervous System
The peripheral nervous system comprises a vast network of nerve fibers extending throughout the human body and interacting with every organ. Peripheral nerves can be further classified as autonomic (supplying sympathetic and parasympathetic nerve signals from the brain to tissue and organs, i.e., fear inducing production of adrenaline) and somatosensory (supplying signals to the brain from tissue and organs, i.e., the sensation of pain). Whether as a root cause or a manifestation of resulting symptoms, these nerves play a role in virtually all diseases.
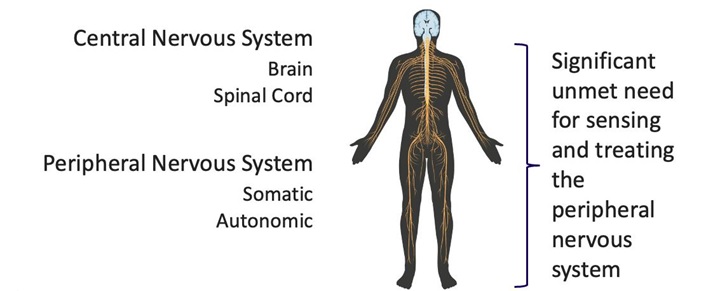
Unfortunately, we believe that very few tools currently exist for the sensing and targeting of nerve fibers within the peripheral nervous system. At Autonomix, our primary goal is to provide a breakthrough in sensing technology that will become an indispensable tool for diagnosing, targeting, and treating disorders relating to the peripheral nervous system. And, although our Company’s name hails from the autonomic subgroup of the peripheral nervous system, our technology is intended for both the autonomic and somatosensory systems and could eventually find uses within the central nervous system.
Exploiting the Vascular Superhighway
The Autonomix system we are developing is primarily catheter based, meaning that our sensing equipment will be delivered to its targeted location via a lumen within the body. While this could include oral, urethral, and other natural openings of the body, our primary focus is using the vasculature, most often arteries, to reach our target. Fortunately, nature has endowed us with “superhighway” access in the form of our arterial structure, as most of the peripheral nerves travel along our arteries. As can be seen in this cross-sectional view of the kidney and renal artery, the web of peripheral nerve fibers (shown in yellow) parallels the renal artery, and this form of nerve pathway development is typical throughout the body.
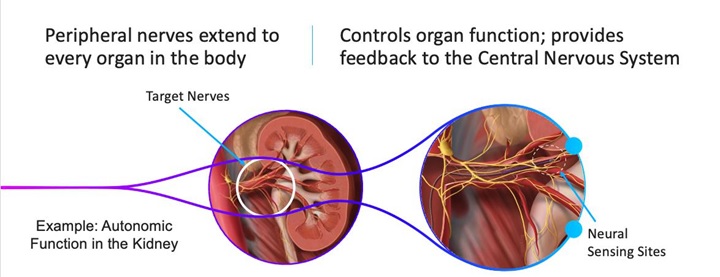
Our sensing catheter has been designed to be introduced by a small incision into an artery (such as the femoral artery) and with a conventional guide wire or sheath be directed to any organ in the body where it will be close enough to the nerve fibers servicing that organ to sense, target and treat the nerves associated with that targeted disorder, and to confirm that the intended treatment was successful.
The Sensing Problem
Although this vascular superhighway has long been utilized for certain catheter-based evaluation and intervention, we believe its use throughout the body has been limited by the lack of adequate sophistication of catheter systems. According to a Markets and Markets report, titled “Electrophysiology Market Global Forecast to 2027” published in February 2023, the global electrophysiology market in terms of revenue was estimated to be $6.8 billion in 2021 and is expected to reach $11.6 billion by 2027. The report cites as a driver for such market the increased incidence of cardiovascular disease and the use of catheters to deliver corrective ablation in the field of cardiology. Most commonly, radio frequency (RF) energy is emitted from inside the walls of the heart or arteries sufficient to ablate (destroy) a cardiomyocyte or nerve within its path. This “transvascular” use of ablation forms the basis for treating atrial fibrillation, for example.
More recently, companies like Medtronic have successfully used transvascular ablation of the nerves surrounding the renal artery to treat refractory hypertension (high blood pressure that has been resistant to standard drug therapy). One of the challenges they face however is that the nerves they are targeting operate at much lower voltage levels than, say, the level emitted by a cardiomyocyte. In cardiology, there are sensing systems capable of sensing down to a level of about 10 to 15 microvolts. That’s more than enough sensitivity to detect (and target) a cardiomyocyte that is emitting 100 microvolts per pulse, but the nerves around the renal artery (and around most peripheral nerve targets throughout the body) are operating at around 1 to 2 microvolts; much too low to be detected by existing sensing technology.
What this means is that the ablation of nerves from within the renal artery is essentially conducted “blind.” Without a sensing system capable of detecting and targeting signals from nerves within the nervous system, clinicians cannot see the nerves causing hypertension in the patient. As a result, they are forced to hypothesize and treat one small area at a time, hoping they hit the desired target without hitting an unintended target. Over-treating the area could relegate the patient to life in a wheelchair by destroying their ability to regulate blood pressure.
The Autonomix Solution
We believe the reason no one has commercialized a sensing system capable of solving this problem is that the physics involved demanded a major technological breakthrough. By their very nature, electrical signals from the body are analog and even though a 10-microvolt signal can be detected and transmitted down the roughly 2 meters of wire required to travel along the catheter, outside the patient, and into the necessary processing equipment shown in this picture below of a typical catheter lab, this isn’t feasible with the 1 to 2 microvolt signal from a typical peripheral nerve. Given the cacophony of other signals emitted throughout the body and by other equipment in the lab as well as degradation of the signal due to the distance traveled along the catheter, these faint signals become lost or are rendered meaningless.
We are seeking to solve this problem through our design, which is still in development, of a proprietary microchip comprised of multiple key components. Each antenna is comprised of two small electrodes that can detect the presence of voltage down to as little as 0.5 microvolts giving us sufficient sensitivity to register the impulse of a nearby nerve bundle that might typically be generating 1.5 to 2.0 microvolts with each impulse. Our current design connects 8 antennae to our proprietary chipset (which is designed to handle up to 16 antennae) where an onboard amplifier and analog to digital converter convert each signal into a robust digital form. The chipset also includes a multiplexer intended to enable the transmission of data from each of the antennae simultaneously down the catheter body to the catheter handle. The Wi-Fi handpiece then transmits this data to a nearby laptop for viewing and analysis by the clinician.
In a typical catheter lab, these signal conversion functions are often carried out by “briefcase” sized devices processing the raw analog signals that must travel the full length of the catheter, outside the patient’s body and then from the patient to the equipment. While this is feasible for higher voltage signals from the heart, the signals from peripheral nerve bundles are often too faint to travel all this distance without loss or corruption and yet the typical catheter lab equipment is far too big to fit inside a catheter. The patented Autonomix solution shrinks these processes down to a microchip small enough to place immediately adjacent to the antennae detecting the signals, greatly reducing the distance the signals must travel. The picture of the “proprietary chipset” below is our actual chipset and is not a rendering.
As shown in the diagram below, this basket antenna array is built from a micro-thin, laser-cut flexible circuit board. We believe the special arrangement of the antennae will make it possible to effectively geolocate the nerve in 3-dimensional space for targeting treatment.
 |
|
Sense, Ablate, Confirm
This sensing system is currently being developed to be deployed alongside a separate radio frequency ablation catheter system for a combined diagnostic and therapeutic solution which will require the use of two separate catheters that will be used during the procedure. However, our longer-term design is intended to combine the two catheters into one device so that the combined system could be capable of sensing, treating (ablating) and confirming successful treatment all with one relatively simple and minimally invasive procedure.
Focus on Pancreatic Cancer Patients
We believe the Autonomix sensing technology has the potential to provide a level of detail and resolution in navigating the peripheral nervous system that until now has simply not been possible. As such, we believe this platform, if shown to be effective, could be applied to a wide range of disorders throughout the body. With that said, our experience tells us that the best way to develop a new technology like this is to narrowly focus on a proof of concept that we think will reflect the capabilities of our system while providing the most expeditious pathway to regulatory clearance, commercialization and revenue generation.
For this reason, we are initially focusing on the treatment of pain associated with pancreatic cancer and we have designed our commercialization efforts around this as our first proposed indication for use.
We believe this is a good choice for several reasons:
Significant Unmet Need
According to a report by The Oncologist, titled “Pancreas Cancer-Associated Pain Management” first published April 22, 2021, “[p]ain is highly prevalent in patients with pancreas cancer,” “90% of these patients reported discussing pain with their health care provider,” and “50% of the respondents reported visits to the emergency room for symptoms related to pain.” One of the tragedies of this condition is that most pancreatic cancer patients have a short time to live and the debilitating pain resulting from the tumor can significantly reduce the quality of that remaining time. Moreover, we believe that prolonged pain can diminish a patient’s will to live, making that remaining time even shorter.
The standard of care treatment usually begins with opioids, but patients often become resistant, and the side effects of chronic opioid use can eventually outweigh the benefits.
The most common alternative method of treatment is a neurolytic celiac plexus blockade (“NCPB”), which is a percutaneous (via needle through the skin) ethanol injection guided by CT scan to attempt to direct the ethanol (which will destroy neural tissue on contact) to the area of the pancreatic tumor and related peripheral nerves. Regardless of this initial targeting, the varied structure of the abdominal cavity leads to the potential for ethanol to either miss the intended target or migrate to unintended areas creating unwanted side effects.
Furthermore, according to a study titled “Neurolytic Celiac Plexus Block for Pain Control in Unresectable Pancreatic Cancer” published by the American Journal of Gastroenterology, 2007, Vol.102 (2), p.430-438, Article 430, meta-analysis from multiple randomized controlled trials suggests that patient benefits from NCPB are only marginally better than opioids and may not be outweighed by the potential risks. The most common side effects are diarrhea, transient hypotension, constipation, nausea and vomiting, and lethargy while rare major adverse events reported in the literature include infectious complications, bowel perforation, intraabdominal hemorrhage, fistula formation, stomach paralysis, partial paralysis of the lower limbs or loss of other motor function, chronic diarrhea, arterial damage, water on the lung, and death.
In contrast, we believe the Autonomix procedure has the potential to represent a safer and more reliable treatment. This has the potential to significantly increase remaining quality of life for pancreatic cancer patients, and in so doing, even potentially extend overall survival.
To begin with, our entire approach is via arterial catheter, inserted in most cases via the femoral or brachial artery. We believe this method of access that should significantly reduce the potential for complications as compared with NCPB. We believe our sensing technology has the potential to identify and target the nerves that are responsible for the pain signal and with the ability to focus the ablative energy on that target, we should have a much greater degree of accuracy, control and reliability as compared with NCPB.
When comparing to the use of opioids, we believe the potential benefits are even more obvious. The Autonomix procedure we are developing is, by design, targeted directly to the nerves responsible for the pain being treated and offers the potential for “one and done” durability, whereas opioids are systemic treatments subjecting the entire body to unnecessary exposure, requiring constant dosing, and inducing debilitating chronic systemic side effects as a consequence.
Beneficial Clinical Trial Dynamics/Expedited Regulatory Process
Despite the significant unmet need, there are very few clinical trials worldwide focused on improving pain management for pancreatic cancer patients and currently none that we are aware of in the proposed location of our planned first-in-human proof of concept study. We believe this means there is limited competition for such patients, making it theoretically easier to recruit for our trial.
At the same time, because we are focusing on palliative care for patients whose lives are being limited by a rare cancer, we believe regulatory authorities are willing to consider lower preclinical hurdles and smaller and simpler trial designs to help encourage trial sponsors to seek improved treatment options. However, these decisions are under the exclusive control of regulatory authorities and there is no guarantee that our trial designs will be approved. If regulatory authorities are willing to consider lower preclinical hurdles and smaller and simpler trial designs, this would translate into lower preclinical and clinical trial cost, as well as shorter completion times. Furthermore, study duration is also shortened by the very nature of the indication and primary efficacy endpoint: reduction of pain associated with pancreatic cancer.
Specifically, we intend for each patient to need only one treatment and we expect we will be able to immediately determine if there is any reduction of pain from our procedure such that an initial indication of pain reduction will likely be provided by patients upon conclusion of that treatment. Although follow up visits will be required to assess continuing safety and durability of efficacy over a span of several months, an initial indication of efficacy will be available almost as quickly as patients are treated . For this reason, we are hopeful that the overall duration of this first trial will be measured in months rather than years, as is often the case for clinical trials with longer treatment durations or where a clinically significant response takes more time to be produced.
Meaningful Commercial Market
Although pancreatic cancer is considered a rare disease, according to the American Cancer Society “Key Statistics for Pancreatic Cancer” (https://www.cancer.org/cancer/types/pancreatic-cancer/about/key-statistics.html), the American Cancer Society estimates in 2023 that approximately 64,000 will be diagnosed with pancreatic cancer in the U.S. on an annual basis and an article in the International Journal of Cancer (Int. J. Cancer. 2021;149:993–1001) indicates that annual new cases in the European Union reached 109,000 in 2019 and are expected to grow. A market analysis published by Precedence Research (https://www.precedenceresearch.com/ pancreatic-cancer-market) reported that the global market for treatment of pancreatic cancer in 2022 was estimated to be $2.2 billion. Published research by The Oncologist, titled “Pancreas Cancer-Associated Pain Management” stated that “90% of patients [with pancreatic cancer] reported discussing pain with their health care provider”. As a point of reference, a one-month course of Abraxane (a commonly prescribed drug for the treatment of pancreatic cancer) has a retail price of more than $10,000. While this should not be considered an indicator of how an Autonomix procedure will ultimately be priced, we believe it reflects the magnitude of potential market size and helps form the basis for expecting a significant revenue opportunity from this indication.
The incidence of pancreatitis, a non-cancerous condition that can also result in chronic pain, is estimated to be as much as three times that of pancreatic cancer. We believe that, if our procedure is cleared for use in treating pancreatic cancer pain, we should be able to eventually expand that clearance to include pain resulting from pancreatitis.
The Potential to Impact Cancer
Recent independent research has indicated that neural pathways may play an insidious role in cancer progression. An article published in Metastatic Cancer: Clinical and Biological Perspectives, titled “Sympathetic Nervous System Regulation of Metastasis” demonstrates that as pancreatic tumors progress to invade the liver (a common occurrence in patients with pancreatic cancer and a significant driver of morbidity) they do so by traveling along local neural pathways. Our development team speculated that disruption of these pathways might have the potential to slow or stop the progression of the primary tumor.
In collaboration with a specialist in pancreatic cancer, we conducted a study in mice to see if ablation (in this study, ethanol ablation was used, similar to its use with NCPB in humans) of the nerve fibers around the pancreas might have an effect on tumor progression. As can be seen in this study summary, there was a reduction in tumor progression in this model. This was a small study, and we can’t be certain that these results are indicative of the potential for impacting tumor progression in humans, but we do see this as encouraging further study and may represent a future opportunity beyond pain management.
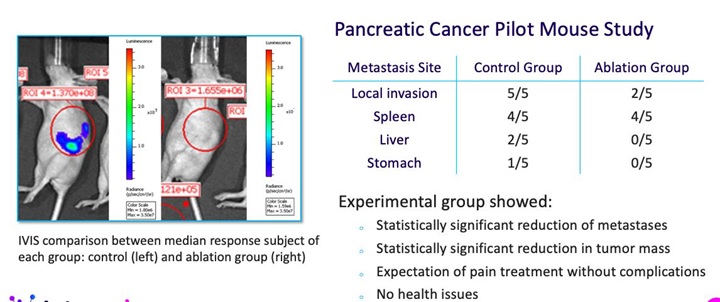
Indicative of Additional Market Potential
We believe pancreatic cancer pain management is a “proof of concept,” and we believe success here will be indicative of the potential of our system in a wide range of disorders where the peripheral nervous system is involved.
Examples of future potential additional uses include renal denervation for treating hypertension, addressing other sources of pain including lower back and other joint locations, Complex Regional Pain Syndrome (“CRPS”), other tumor related pain, and pelvic pain, pulmonary disorders such as chronic obstructive pulmonary disease, and urinary tract and digestive disorders, and enabling more targeted treatments in cardiology, just to name a few.
A Markets and Markets report, titled “Electrophysiology Market Global Forecast to 2027” published in February 2023 describes the global electrophysiology (EP) market as representing approximately $6.8 billion in 2021 in annual global revenue, and is expected to reach $11.6 billion by 2027. The vast majority of this market today is represented by cardiology related diagnosis and intervention. Our vision for the Autonomix technology is to help expand electrophysiology well beyond cardiology to include nearly all reaches of the peripheral nervous system and we believe doing so will ultimately result in a market opportunity much greater than the current projections for the EP.
We believe enabling targeted transvascular treatment of pain will enable us to access the $78 billion pain management market, as cited in the Mordor Intelligence Pain Management Market Industry Report (https://www.mordorintelligence.com/industry-reports/pain-management-market). Additionally, if we are able to facilitate a safer, more targeted method for renal denervation we may be able to access the $25 billion hypertension market, as indicated by Polaris Market Research (https://www.polarismarketresearch.com/industry-analysis/global-hypertension-drug-market). When additional indications such as COPD, irritable bowl syndrome, and overactive bladder are included, we believe the Autonomix platform has the potential to address more than $100 billion in market opportunities.
Commercialization Plan
Regulatory Pathway
We believe, the most likely approval pathway for our technology is referred to as “de novo” premarket notification. This is differentiated from the more common “510(k)” pathway, which is only applicable when there is a clear “predicate” device already on the market (doing the same thing in substantially the same way) and from the lengthier “PMA” process when there is no precedent at all for a technology. In our case, both sensing and ablation have established precedence, just not at this level of sensitivity or in our targeted indications.
Whether in the United States or EU, we must demonstrate that our technology is safe and effective. The safety standard is ultimately met through a combination of animal studies, independent laboratory testing, a design history file documenting compliance with established standards and, ultimately, human clinical trials. Many of these requirements are staged such that not all must be met on the front end of development. In addition, efficacy must be based on a sound scientific rationale and ultimately demonstrated in a human clinical trial.
Human trials are often designed to begin with a Proof of Concept (“PoC”); the US Food and Drug Administration (“FDA”) sometimes refers to these as Early Feasibility Studies (“EFS”) and then progress to a “Pivotal” or approval, trial. The design and endpoints of pivotal trials are often negotiated with the relevant regulatory authority (i.e., FDA in the United States, EMA or country-specific Competent Authority (“CA”) in Europe). Our regulatory package for authorization to conduct our first-in-human clinical trial was approved by the Ethics Committee (“EC”) at our intended clinical site hospital outside the United States. This approval allowed the clinical trial to begin The regulatory package submitted included not only a detailed clinical protocol for conducting the study, but also an extensive Investigator’s Brochure (“IB”) setting forth details about the equipment to be used, historical safety of human procedures conducted with this equipment and details of our animal studies using this equipment for the first time in the area of the pancreas.
We plan to present the relevant data from this study to the FDA in a pre-submission meeting to request “Breakthrough Status” in an effort to minimize the clinical requirements for clearance in the United States. The first trial is not designed to replace the trial that will be required by the FDA to support our submissions for clearance in the United States, but rather to potentially impact the size of that required trial. There is no assurance that the FDA will accept the results of the data we obtain from our clinical trials performed outside of the United States.
According to an article by Applied Clinical Trials, titled “Medical Device Development: U.S. and EU Differences” published August 1, 2006 “[t]he way in which devices are regulated in the EU is very different from the way they are regulated in the United States,… [which] has introduced significant differences in time-to-market approval for the United States versus the EU, particularly in the case of high-risk Class III and Class IIb implantable devices.” While this is changing based on the advent of a new EU regulation called Medical Device Regulation (“MDR”) that is expected to make the EU process more like the FDA process, MDR is being rolled out country by country. We believe the approval process in some EU countries for utilizing CE marked devices off label is less demanding than in the US. As a result, we have decided to conduct the PoC in Europe instead of the US.
Once the PoC is established, however, there are compelling reasons to focus the approval process first and foremost in the US. One reason is that therapeutic procedures in the US will usually command higher prices and once those prices are set, EU pricing authorities will often index off of the US price. Another is that product launches are usually easier in the US where a single sales force can serve the entire region (as opposed to country-specific distribution teams in the EU) and where one regulatory standard applies across the board.
The development of the PoC data forms the basis for two FDA-related processes. First is the request of a “Pre-Submission” meeting to discuss the overall regulatory strategy, the primary focus of which is to agree on a pivotal testing protocol. Assuming promising PoC data, the second is to submit a request for “Breakthrough” status based on the significant unmet need. Breakthrough status affords us expedited access to FDA and a higher level of proactive interaction that may support faster approval.
Upon completion of the Pivotal Clinical Trial, we believe we will then be in a position to submit our de novo application to the FDA, the review of which is expected to require approximately 150 days. If we able to receive FDA clearance (applications like this are not technically “approved” but rather “allowed” or “cleared”), of which there is no assurance, we currently estimate such clearance would occur in the first half of 2027. The foregoing timeline is not a guarantee and is subject to many of the risks and uncertainties disclosed in this prospectus and is subject to change.
Technology Development
Commercialization of our technology can be thought of in three distinct phases: (1) sensing, (2) ablation, and (3) the combination of these two technologies into an integrated device. We believe that commercial success can be achieved with either of the two technologies and is not dependent upon successful integration (meaning two distinct systems, one for sensing and one for ablation could also be viable, even if not optimal).
Similarly, our clinical development plan reflects the fact that the sensing and ablation systems are at different stages of development. Extensive testing in pigs (whose abdominal structure is considered similar to humans) has demonstrated that our sensing technology is capable of locating and targeting individual nerves around the renal artery. Considering the similarity in anatomy, both between pigs and humans and between the renal artery (supplying the kidneys) and the celiac and related arteries supplying the pancreas, we believe our sensing system may also be effective in humans and that the primary remaining risk relating to the sensing system is commercial execution.
Specifically, we believe one of the most demanding aspects of our commercialization plan will be scaling up from our existing sensing prototype to a robust commercial version. Today, our sensing device is hand built and includes a combination of hand-crafted and 3D printed parts, but we are actively engaged in the development of a more robust version which will meet requirements to be used in human clinical trials. We will divide this device into two subsets: (a) an electronics package (subassembly) that relies on semi-automated production of printed flexible circuit boards and electrical leads that is supplied to (b) a qualified catheter production process that will be contracted to a catheter production facility already experienced and certified in the “art” of catheter assembly. We expect the human clinical version to be completed by mid-2025 and the commercial scale up process to be completed by mid-2027, although there is no assurance that we will be able to meet such timeline.
Regarding the ablation system, safe and reliable off-the-shelf RF systems are currently available for use in cardiology and other electrophysiology indications. We are currently using one of these existing systems “off label” (in an area of the body for which the system is not yet approved by the relevant regulatory agency) in our proof-of-concept study to ablate the nerves near the pancreas of pancreatic cancer patients to demonstrate, for the first time ever, that transvascular ablation of those nerves may reduce pain. The regulatory package submitted includes not only a detailed clinical protocol for conducting the study, but also an extensive Investigator’s Brochure (IB) setting forth details about the equipment to be used, historical safety of human procedures conducted with this equipment and details of our animal studies using this equipment for the first time in the area of the pancreas. To be clear, this first PoC trial is being conducted without the benefit of our sensing system because it has not yet been cleared by the FDA for use in humans.
The fact that this first PoC is essentially being performed “blind” (as all denervation procedures are currently done) simply means that it will not be as accurate as it could be. However, we believe if we successfully demonstrate that transvascular ablation is capable of mitigating pancreatic cancer pain, this would be a medical “first” and would represent an important breakthrough for the electrophysiology community. Likewise, we believe it will help support a request to the FDA to be granted “Breakthrough” status, which could accelerate our future commercialization efforts.
Also, what we are learning in the trial, about how to optimize ablation catheters for use outside the cardiology space, is being incorporated into the design of a customized RF catheter design for use in our own therapeutic device.
It is possible to receive clearance from the FDA (and EMA) based on a prototype system that is not optimized for manufacturing and for the necessary design for manufacturability process to be running in the background with the goal of having the commercial version ready for launch shortly after the prototype platform is FDA cleared. We would then use our existing de novo cleared device as our own predicate for 510(k) clearance of the commercial version.
Our plan is then to launch the commercial version of our system, most likely to a controlled region or list of KOLs (Key Opinion Leaders) to debug and optimize the commercial strategy before committing to a national/global launch.
Accordingly, our planning considers both the possibility of a stand-alone commercial launch or a licensing arrangement with a larger player. As we get closer to an actual FDA device clearance, our strategy approach will be reviewed and adjusted for practicality. The Autonomix management team, however, has experience with both approaches.
The following graphic sets forth our planned development timeline, however, these timeframes are not guarantees and this timeline is subject to many of the risks and uncertainties disclosed in this prospectus and is subject to change:
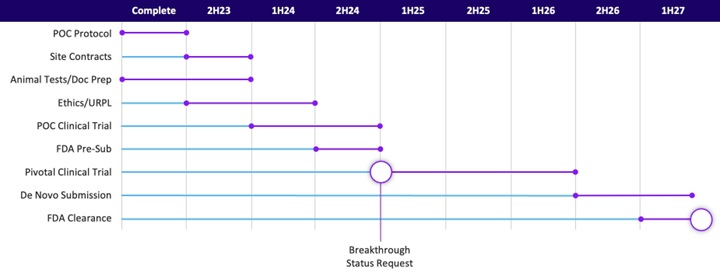
Revenue Model
We envision our device revenue model on several levels: (a) purely as a targeted therapy for disorders associated with pancreatic tumors (and pancreatitis), (b) as a standalone sensing technology for broad based diagnostics use and/or use in conjunction with other ablation systems or (c) as a combined sensing and ablation system targeting a wide array of disorders throughout the body.
There are four key elements of our device that will be provided to the user (hospital) to facilitate our technology: (1) a disposable sensing catheter with a handpiece (that connects to the catheter and could be partially or entirely disposable), (2) an RF catheter designed to reach the target nerves in the peripheral nervous system, (3) an RF energy source to power our custom RF ablation catheter, and (4) a user interface comprised primarily of software (translating data received from the catheter microchip and activating ablative energy in integrated systems) that could reside on existing hospital personal computer, or PC, systems or a dedicated PC system.
In general, we believe that hospitals place a high premium on disposability (maximizes patient safety, avoids complications of on-site sterilization) and that, if we can avoid the need for a significant outlay in “capital equipment” we should, since high-dollar capital equipment authorizations often involve additional bureaucracies within the hospital and complicate the sell-in process.
An important benefit of the Autonomix platform will be that it will rely on catheter systems and techniques that are familiar to interventional radiologists (our expected primary users) and should require a minimum of training for successful use.
Our selection of radio frequency as an energy source was made based upon what we believe is the well-understood status of the technology within the industry and the FDA. Additionally, we have found in pre-clinical testing that this energy source appears to provide a very effective method of ablating neural tissue. The software required in our device’s user interface will likely be provided at no or a nominal charge.
For the reasons described above, we believe the primary revenue model for Autonomix will be the sale of single-use disposable catheters to existing hospital catheter labs and that revenue volume will likely be a direct function of the number of procedures performed.
Intellectual Property
Patents and Pending Patent Applications
The Company has 18 patent families (representing various inventions relating to different aspects of its technology) comprising 87 issued patents (37 in the US thus far) and 42 pending patent applications. All patents and patent applications were originated by our co-founders, Mr. Landy Toth and/or Dr. Robert Schwartz, with filing dates ranging from 2012 through 2024.
The following table shows our material patents and the expiration dates (assuming maintenance/annuity fees are paid as required) as of October 31, 2024:
Issued Patents
|
Patent No. |
Jurisdiction |
Title |
Patent Expiration Date |
|
2013211951 |
Australia |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
2,862,862 |
Canada |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
| 3,151,885 | Canada | Controlled Sympathectomy and Micro-Ablation Systems and Methods | 1/25/2033 |
|
ZL201380016637.3 |
China |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
ZL201710440397.X |
China |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
2804527 |
European Patent Ratified in France, Germany, Ireland, Netherlands, UK |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
497881 |
India |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
6,552,824 |
Japan |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
347625 |
Mexico |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
372926 |
Mexico |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
11201406006X |
Singapore |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
10,470,684 |
United States of America |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
11/10/2034 |
|
9,649,064 |
United States of America |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
4/28/2033 |
|
10,022,085 |
United States of America |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
11,013,459 |
United States of America |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
6/30/2033 |
|
2013267672 |
Australia |
Endoscopic Sympathectomy Systems and Methods |
5/28/2033 |
|
2,874,620 |
Canada |
Endoscopic Sympathectomy Systems and Methods |
5/28/2033 |
|
2852339 |
European Patent Ratified in France; Germany; Ireland: Netherlands; UK |
Endoscopic Sympathectomy Systems and Methods |
5/28/2033 |
|
436507 |
India |
Endoscopic Sympathectomy Systems and Methods |
5/28/2033 |
|
504735 |
India |
Endoscopic Sympathectomy Systems and Methods |
5/28/2033 |
|
11201407873R |
Singapore |
Endoscopic Sympathectomy Systems and Methods |
5/28/2033 |
|
9,968,790 |
United States of America |
Endoscopic Sympathectomy Systems and Methods |
7/14/2033 |
|
10,226,633 |
United States of America |
Endoscopic Sympathectomy Systems and Methods |
5/28/2033 |
|
11,344,731 |
United States of America |
Endoscopic Sympathectomy Systems and Methods |
5/28/2033 |
| 12,053,634 | United States of America | Endoscopic Sympathectomy Systems and Methods | 5/28/2033 |
|
2013274158 |
Australia |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
6/13/2033 |
|
2,876,080 |
Canada |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
6/13/2033 |
|
2,861,145 |
European Patent Ratified in France; Germany; Ireland; Netherlands; UK |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
6/13/2033 |
|
532368 |
India |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
6/13/2023 |
|
6,335,888 |
Japan |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
6/13/2033 |
|
6,672,370 |
Japan |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
6/13/2033 |
|
11201408219T |
Singapore |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
6/13/2033 |
|
10,206,616 |
United States of America |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
9/10/2034 |
|
11,564,616 |
United States of America |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
8/18/2033 |
|
2013337879 |
Australia |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
10/31/2033 |
|
2,889,674 |
Canada |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
10/31/2033 |
|
2,914,334 |
European Patent Ratified in France; Germany; Ireland; Netherlands; UK |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
10/31/2033 |
|
406600 |
India |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
10/31/2033 |
|
11201503472P |
Singapore |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
10/31/2033 |
|
9,956,034 |
United States of America |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
10/18/2034 |
|
10,905,495 |
United States of America |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
11/6/2033 |
|
12,0111,214 |
United States of America |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
5/13/2034 |
|
2013354932 |
Australia |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
12/9/2033 |
|
2,892,449 |
Canada |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
12/9/2033 |
|
388850 |
India |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
12/9/2033 |
|
11201504119V |
Singapore |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
12/9/2033 |
|
10,674,963 |
United States of America |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
6/1/2036 |
|
10,363,359 |
United States of America |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
2/25/2036 |
|
11,478,582 |
United States of America |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
8/2/2034 |
|
2014241205 |
Australia |
Neurological Traffic and Receptor Evaluation and Modification: Systems and Methods |
3/27/2034 |
|
2,907,625 |
Canada |
Systems and Methods for Neurological Traffic and/or Receptor Functional Evaluation and/or Modification |
3/27/2034 |
|
2,978,372 |
European Patent Ratified in Belgium; France; Germany; Ireland; Italy; Netherlands; Spain; UK |
Systems and Methods for Neurological Traffic and/or Receptor Functional Evaluation and/or Modification |
3/27/2034 |
|
480497 |
India |
System for Neurological Traffic and Receptor Evaluation and Modification |
3/27/2034 |
|
11201507936U |
Singapore |
Neurological Traffic and Receptor Functional Evaluation and Modification |
3/27/2034 |
|
10,004,458 |
United States of America |
Systems and Methods for Neurological Traffic and/or Receptor Functional Evaluation and/or Modification |
1/16/2035 |
|
10,765,370 |
United States of America |
Systems and Methods for Neurological Traffic and/or Receptor Functional Evaluation and/or Modification |
4/10/2034 |
|
11,589,820 |
United States of America |
Systems and Methods for Neurological Traffic and/or Receptor Functional Evaluation and/or Modification |
12/4/2034 |
|
2014337552 |
Australia |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
10/14/2034 |
|
2,926,088 |
Canada |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
10/14/2034 |
|
443928 |
India |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
10/14/2034 |
|
364705 |
Mexico |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
10/14/2034 |
|
10,143,419 |
United States of America |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
2/6/2035 |
|
11,272,877 |
United States of America |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
2/6/2035 |
| 12,064,256 | United States of America | Systems and Methods for Treating Cancer and/or Augmenting Organ Function | 3/6/2035 |
|
10,136,944 |
United States of America |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
5/24/2035 |
|
11,382,687 |
United States of America |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
4/3/2035 |
|
2015302050 |
Australia |
ANS Assessment Systems, Kits, and Methods |
8/7/2035 |
|
2,957,766 |
Canada |
ANS Assessment Systems, Kits, and Methods |
8/7/2035 |
|
3,177,201 |
European Patent Ratified as Unitary Patent and in Ireland and UK |
ANS Assessment Systems, Kits, and Methods |
8/7/2035 |
|
369552 |
India |
ANS Assessment Systems, Kits, and Methods |
8/7/2035 |
|
11201701018P |
Singapore |
ANS Assessment Systems, Kits, and Methods |
8/7/2035 |
|
10,791,924 |
United States of America |
ANS Assessment Systems, Kits, and Methods |
9/2/2037 |
|
11,883,103 |
United States of America |
ANS Assessment Systems, Kits, and Methods |
10/13/2036 |
|
ZL201680068094.3 |
China |
Controlled and Precise Treatment of Cardiac Tissues |
10/20/2036 |
|
3,364,869 |
European Patent Ratified as Unitary Patent and in Ireland and UK |
Controlled and Precise Treatment of Cardiac Tissues |
10/20/2036 |
|
11,154,239 |
United States of America |
Controlled and Precise Treatment of Cardiac Tissues |
5/28/2038 |
|
11,521,738 |
United States of America |
Medical Devices with Circuitry for Capturing and Processing Physiological Signals |
4/14/2039 |
|
11,848,078 |
United States of America |
Medical Devices with Circuitry for Capturing and Processing Physiological Signals |
10/17/2038 |
|
9,730,639 |
United States of America |
Elongated Conductors and Methods of Making and Using the Same |
5/19/2036 |
|
10,092,241 |
United States of America |
Elongated Conductors and Methods of Making and Using the Same |
5/19/2036 |
|
10,238,340 |
United States of America |
Elongated Conductors and Methods of Making and Using the Same |
5/19/2036 |
|
10,485,484 |
United States of America |
Elongated Conductors and Methods of Making and Using the Same |
5/19/2036 |
|
10,869,635 |
United States of America |
Elongated Conductors and Methods of Making and Using the Same |
5/19/2036 |
|
11,445,979 |
United States of America |
Elongated Conductors and Methods of Making and Using the Same |
5/19/2036 |
| 12,070,334 | United States of America | Elongated Conductors and Methods of Making and Using the Same | 5/19/2036 |
|
107924736 |
China |
Elongated Conductors and Methods of Making and Using the Same |
5/19/2036 |
|
10,874,830 |
United States of America |
Smart Torquer and Methods of Using the Same |
6/14/2037 |
The following table shows our material pending patent applications as of October 31, 2024 (note that no expiration dates are specified for the pending patent applications since, in certain jurisdictions, e.g., United States of America, the patent expiration date is calculated at the time of issue/grant):
Pending Patent Applications
|
Application No. |
Filing Date |
Jurisdiction |
Title |
|
3239581 |
5/24/2024 |
Canada |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
|
21168169.7 |
5/13/2021 |
European Patent Office |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
|
16/591,126 |
10/2/2019 |
United States of America |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
|
17/325,833 |
5/20/2021 |
United States of America |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
|
3158197 |
5/4/2022 |
Canada |
Endoscopic Sympathectomy Systems and Methods |
|
18/771,099 |
7/12/2024 |
United States of America |
Endoscopic Sympathectomy Systems and Methods |
|
3184524 |
12/15/2022 |
Canada |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
|
202448028613 |
4/8/2024 |
India |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
|
18/079,361 |
12/12/2022 |
United States of America |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
|
3183802 |
12/9/2022 |
Canada |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
|
18/656,031 |
5/6/2024 |
United States of America |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
|
3151434 |
3/9/2022 |
Canada |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
|
EP24170108.5 |
4/12/2024 |
European Patent Office |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
|
16/855,080 |
4/22/2020 |
United States of America |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
|
17/971,202 |
10/21/2022 |
United States of America |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
|
3,205,904 |
7/10/2023 |
Canada |
Systems and Methods for Neurological Traffic and/or Receptor Functional Evaluation and/or Modification |
|
19159405 |
2/26/2019 |
European Patent Office |
Systems and Methods for Neurological Traffic and/or Receptor Functional Evaluation and/or Modification |
|
202348081808 |
12/1/2023 |
India |
Systems and Methods for Neurological Traffic and/or Receptor Functional Evaluation and/or Modification |
|
18/104,460 |
2/1/2023 |
United States of America |
Systems and Methods for Neurological Traffic and/or Receptor Functional Evaluation and/or Modification |
|
3182302 |
11/28/2022 |
Canada |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
|
22175617.4 |
5/26/2022 |
European Patent Office |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
|
18/772/819 |
7/15/2024 |
United States of America |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
|
17/833,964 |
6/7/2022 |
United States of America |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
|
23182480.6 |
6/29/2023 |
European Patent Office |
ANS Assessment Systems, Kits, and Methods |
|
3,210,898 |
9/1/2023 |
Canada |
ANS Assessment Systems, Kits, and Methods |
|
10202300654X |
3/10/2023 |
Singapore |
ANS Assessment Systems, Kits, and Methods |
|
18/543,538 |
12/18/2023 |
United States of America |
ANS Assessment Systems, Kits, and Methods |
|
23171646.5 |
5/4/2023 |
European Patent Office |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
|
15864478.1 |
7/4/2017 |
European Patent Office |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
|
202210497355 |
5/9/2022 |
China |
Controlled and Precise Treatment of Cardiac Tissues |
|
23170175.6 |
4/26/2023 |
European Patent Office |
Controlled and Precise Treatment of Cardiac Tissues |
|
17/501,502 |
10/14/2021 |
United States of America |
Controlled and Precise Treatment of Cardiac Tissues |
|
18868117.5 |
4/17/2020 |
European Patent Office |
Medical Devices with Circuitry for Capturing and Processing Signals |
|
18/387,585 |
11/7/2023 |
United States of America |
Medical Devices with Circuitry for Capturing and Processing Signals |
|
PCT/US2023/029193 |
8/1/2023 |
Patent Cooperation Treaty (PCT)/ United States of America, Receiving Office |
Medical Devices Configured for Therapeutic Electroporation of Biologic Tissues |
|
18/779,387 |
7/22/2024 |
United States of America |
Elongated Conductors and Methods of Making and Using the Same |
|
16803986.5 |
11/29/2017 |
European Patent Office |
Elongated Conductors and Methods of Making and Using the Same |
|
16/951,317 |
11/18/2020 |
United States of America |
Smart Torquer and Methods of Using the Same |
|
16854068 |
4/9/2018 |
European Patent Office |
Smart Torquer and Methods of Using the Same |
|
63/636,467 |
4/19/2024 |
United States of America |
Medical Devices Configured for Temperature-Controlled Therapeutic Electroporation |
|
63/636,471 |
4/19/2024 |
United States of America |
Medical Devices Configured for Therapeutic Electroporation with Adjustable Sections of an Elongate Member |
|
63/636,477 |
4/19/2024 |
United States of America |
Medical Device Guide Sheaths with Return Electrodes for Bipolar Therapeutic Electroporation |
Trademarks
The Company has one trademark for the mark “AUTONOMIX” in the United States, Australia, China, the European Union, India, Japan, Mexico, and Singapore. The registrations of these trademarks are effective for varying periods of time and may be renewed periodically provided we comply with all applicable renewal requirements, including, where necessary, the continued use of the trademarks in the applicable jurisdictions in connection with certain goods and services.
License Agreement
In December 2021, we granted a company affiliated with certain early investors in the Company a exclusive worldwide license to our technology for use in the diagnosis and treatment of cardiovascular conditions and/or hypertension through renal denervation-based methods. During the term of the license agreement, we did not receive any fees from the licensee, and did not generate any revenue under the license agreement. On July 7, 2023, we entered into an Exclusive License Termination Agreement (the “Termination Agreement”) with the Licensee in exchange for the issuance, upon the closing of our IPO within one year of the agreement’s execution, of a warrant to purchase shares of the Company for a variable number of shares based on a value of $8.0 million. Upon the closing of our IPO on January 29, 2024, we issued the licensee a warrant to purchase 80,000 shares which were issued at an assumed valuation of $100.00 per share for a fixed value of $8.0 million. The warrants are exercisable at a price of $0.02 per share and may be exercised any time after the issuance date, subject to a beneficial ownership limitation, and expire five years from the original issuance. The warrants provide dividend rights. The shares underlying the warrant are subject to a lockup agreement for a period of six months after the closing of the IPO with respect to 12.5% of the shares issued and twelve months after the closing of the IPO for the remainder of the shares. One of our directors, David Robins, holds a 19.5% interest in the company receiving the warrant.
Competition
The electrophysiology market is served by a number of very large players including, for example, Medtronic and Boston Scientific, all of whom have substantially greater resources than Autonomix.
While the current standards of care for treating pancreatic cancer pain are comprised largely of generic drugs and injections, we are not aware of any person or company working on a transvascular method for treating this indication. Although competitors like Medtronic are expanding the use of transvascular ablation techniques, we are not aware of anyone developing a sensing technology similar in capability to our technology.
Recent tests using Endoscopic Ultrasound-Guided Ablation to treat pancreatic cancer pain have claimed outcomes that may be better than NCPB, however this is still a percutaneous technique that we believe involves similar inherent risks of infection and internal damage as does NCPB. Conversely, we believe these risks are significantly reduced using a transvascular approach such as the Autonomix technology.
Description of Property
The Company leases office space as its headquarters in Texas and has access to a research and development facility in Pennsylvania. We believe our facilities are sufficient to meet our current needs and that suitable space will be available as and when needed.
Employees
As of October 1, 2024, we have 10 individuals providing services to the Company, six on a full-time basis and four on a part-time basis. Accordingly, a high percentage of the work performed for our development projects is conducted by qualified part-time staff and independent contractors.
Legal Proceedings
From time to time, we may become involved in litigation matters relating to claims arising from the ordinary course of business. While the results of such claims and legal actions cannot be predicted with certainty, our management does not believe that there are claims or actions, pending or threatened against us, the ultimate disposition of which would have a material adverse effect on our business, results of operations, financial condition, or cash flows.
Regulation of Our Business
Our product candidate and operations are subject to extensive and rigorous regulation by the U.S. Food and Drug Administration (“FDA”), under the Federal Food, Drug, and Cosmetic Act (“FDCA”), and it’s implementing regulations, guidance documentation, and standards. Our sensing device and catheters are regulated by the FDA as medical devices. The FDA regulates the design, development, research, testing, manufacturing, safety, labeling, storage, record keeping, promotion, distribution, sale and advertising of medical devices in the United States to ensure that medical products distributed domestically are safe and effective for their intended uses. The FDA also regulates the export of medical devices manufactured in the United States to international markets. Any violations of these laws and regulations could result in a material adverse effect on our business, financial condition and results of operations. In addition, if there is a change in law, regulation or judicial interpretation, we may be required to change our business practices, which could have a material adverse effect on our business, financial condition and results of operations.
Unless an exemption applies, before we can commercially distribute medical devices in the United States, we must obtain, depending on the type of device, either prior premarket clearance or premarket approval (PMA), from the FDA. The FDA classifies medical devices into one of three classes:
|
• |
Class I devices, which are subject to only general controls (e.g., labeling, medical devices reporting, and prohibitions against adulteration and misbranding) and, in some cases, to the premarket clearance requirements; |
|
• |
Class II devices, generally requiring premarket clearance before they may be commercially marketed in the United States; and |
|
• |
Class III devices, consisting of devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a predicate device, generally requiring submission of a PMA supported by clinical trial data. |
Our current product candidates are all class II devices and will require submission of a premarket notification.
510(k) Clearance Pathway
When a 510(k) clearance is required, we must submit a premarket notification demonstrating that our proposed device is substantially equivalent to a previously cleared 510(k) device or a device that was in commercial distribution before May 28, 1976 for which the FDA has not yet called for the submission of PMAs. By regulation, the FDA is required to clear or deny a 510(k) premarket notification within 90 days of submission of the application. As a practical matter, clearance may take longer. The FDA may require further information, including clinical data, to make a determination regarding substantial equivalence.
Any modification to a 510(k)-cleared device that would constitute a major change in its intended use, or any change that could significantly affect the safety or effectiveness of the device, requires a new 510(k) clearance and may even, in some circumstances, require a PMA, if the change raises complex or novel scientific issues or the product has a new intended use. The FDA requires every manufacturer to make the determination regarding the need for a new 510(k) submission in the first instance, but the FDA may review any manufacturer's decision.
PMA Pathway
A PMA must be submitted to the FDA if the device cannot be cleared through the 510(k) process. A PMA must be supported by extensive data, including but not limited to, technical, preclinical, clinical trials, manufacturing and labeling to demonstrate to the FDA's satisfaction the safety and effectiveness of the device for its intended use. During the review period, the FDA will typically request additional information or clarification of the information already provided. Also, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. The FDA may or may not accept the panel's recommendation. In addition, the FDA will generally conduct a pre-approval inspection of the manufacturing facility or facilities to ensure compliance with the QSRs.
New PMAs or PMA supplements are required for modifications that affect the safety or effectiveness of the device, including, for example, certain types of modifications to the device's indication for use, manufacturing process, labeling and design. PMA supplements often require submission of the same type of information as a PMA, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA and may not require as extensive clinical data or the convening of an advisory panel.
de novo Classification
Medical device types that the FDA has not previously classified as Class I, II or III are automatically classified into Class III regardless of the level of risk they pose. The FDA Modernization Act of 1997 established a new route to market for low to moderate risk medical devices that are automatically placed into Class III due to the absence of a predicate device, called the “Request for Evaluation of Automatic Class III Designation,” or the de novo classification procedure. This procedure allows a manufacturer whose novel device is automatically classified into Class III to request down-classification of its medical device into Class I or Class II on the basis that the device presents low or moderate risk, rather than requiring the submission and approval of a PMA application. Prior to the enactment of the FDA Safety and Innovation Act of 2012, or the FDASIA, a medical device could only be eligible for de novo classification if the manufacturer first submitted a 510(k) premarket notification and received a determination from the FDA that the device was not substantially equivalent. FDASIA streamlined the de novo classification pathway by permitting manufacturers to request de novo classification directly without first submitting a 510(k) premarket notification to the FDA and receiving a not substantially equivalent determination. Under FDASIA, the FDA is required to classify the device within 120 days following receipt of the de novo application. If the manufacturer seeks reclassification into Class II, the manufacturer must include a draft proposal for special controls that are necessary to provide a reasonable assurance of the safety and effectiveness of the medical device. In addition, the FDA may reject the reclassification petition if it identifies a legally marketed predicate device that would be appropriate for a 510(k) or determines that the device is not low to moderate risk or that general controls would be inadequate to control the risks and special controls cannot be developed.
Clinical Trials
Clinical trials are generally required to support a PMA application and are sometimes required for 510(k) or de novo clearance. Such trials generally require an investigational device exemption application, or IDE, approved in advance by the FDA for a specified number of patients and study sites, unless the product is deemed a nonsignificant risk device eligible for more abbreviated IDE requirements. Clinical trials are subject to extensive monitoring, record keeping and reporting requirements. Clinical trials must be conducted under the oversight of an institutional review board, or IRB, for the relevant clinical trial sites and must comply with FDA regulations, including but not limited to those relating to good clinical practices. To conduct a clinical trial, we also are required to obtain the patients' informed consent in form and substance that complies with both FDA requirements and state and federal privacy and human subject protection regulations. We, the FDA or the IRB could suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits. Even if a trial is completed, the results of clinical testing may not adequately demonstrate the safety and efficacy of the device or may otherwise not be sufficient to obtain FDA approval to market the product in the U.S. Similarly, in Europe the clinical study must be approved by a local ethics committee (EC) and in some cases, including studies with high-risk devices, by the ministry of health in the applicable country.
Pervasive and Continuing Regulation
After a device is placed on the market, numerous regulatory requirements apply. These include:
|
• |
Product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action; |
|
• |
Quality System Regulation, or QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process; |
|
• |
labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label use or indication; |
|
• |
clearance of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use of one of our cleared devices; |
|
• |
approval of product modifications that affect the safety or effectiveness of one of our approved devices; |
|
• |
medical device reporting regulations, which require that manufacturers comply with FDA requirements to report if their device may have caused or contributed to a death or serious injury, or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of the device or a similar device were to recur; |
|
• |
post-approval restrictions or conditions, including post-approval study commitments; |
|
• |
post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device; |
|
• |
the FDA's recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations; |
|
• |
regulations pertaining to voluntary recalls; and |
|
• |
notices of corrections or removals. |
Advertising and promotion of medical devices, in addition to being regulated by the FDA, are also regulated by the Federal Trade Commission and by state regulatory and enforcement authorities. Recently, promotional activities for FDA-regulated products of other companies have been the subject of enforcement action brought under healthcare reimbursement laws and consumer protection statutes. In addition, under the federal Lanham Act and similar state laws, competitors and others can initiate litigation relating to advertising claims. In addition, we are required to meet regulatory requirements in countries outside the U.S., which can change rapidly with relatively short notice. If the FDA determines that our promotional materials or training constitutes promotion of an unapproved use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions.
Furthermore, our products could be subject to voluntary recall if we or the FDA determine, for any reason, that our products pose a risk of injury or are otherwise defective. Moreover, the FDA can order a mandatory recall if there is a reasonable probability that our device would cause serious adverse health consequences or death.
The FDA has broad post-market and regulatory enforcement powers. Once we have a marketed product, we will be subject to unannounced inspections by the FDA to determine our compliance with the QSR and other regulations, and these inspections may include the manufacturing facilities of some of our subcontractors. Failure by us or by our suppliers to comply with applicable regulatory requirements can result in enforcement action by the FDA or other regulatory authorities, which may result in sanctions including, but not limited to:
|
• |
untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
|
• |
unanticipated expenditures to address or defend such actions |
|
• |
customer notifications for repair, replacement, refunds; |
|
• |
recall, detention or seizure of our products; |
|
• |
operating restrictions or partial suspension or total shutdown of production; |
|
• |
refusing or delaying our requests for premarket clearance or premarket approval of new products or modified products; |
|
• |
operating restrictions; |
|
• |
withdrawing premarket clearances or PMA approvals that have already been granted; |
|
• |
refusal to grant export approval for our products; or |
|
• |
criminal prosecution. |
Glossary
Unless we otherwise indicate, or unless the context requires otherwise, any references in this prospectus to the following medical terms have the respective meanings set forth below:
ablation: the removal or destruction of a body part or tissue or its function. This is often conducted with energy-based devices utilizing radio frequency or pulsed electrical field energy.
cardiomyocyte: the cell responsible for the contraction of the heart.
catheter: a thin tube made from medical grade materials serving a broad range of functions; catheters are medical devices that can be inserted in the body to treat diseases or perform a surgical procedure.
celiac plexus: also known as the solar plexus because of its radiating nerve fibers, is a complex network of nerves located in the abdomen, near where the celiac trunk, and other arteries branch from the abdominal aorta.
electrophysiology: the branch of physiology that deals with the electrical phenomena associated with nervous and other bodily activity.
hypertension: high blood pressure.
microvolt: one millionth of a volt.
palliative care: specialized medical care for people living with a serious illness focused on providing relief from the symptoms and stress of serious illness.
renal artery: the main blood vessel that supplies blood to a kidney.
transvascular: across the wall of a blood vessel (or similar vessel).
unresectable: unable to be removed with surgery.
Available Information
Our Internet address is www.autonomix.com. On this website, we post the following filings as soon as reasonably practicable after they are electronically filed with or furnished to the SEC: our Annual Reports on Form 10-K; our Quarterly Reports on Form 10-Q; our Current Reports on Form 8-K; our proxy statements related to our annual stockholders’ meetings; and any amendments to those reports or statements. All such filings are available on our website free of charge. The charters of our audit, nominating and governance and compensation committees and our Code of Business Conduct and Ethics Policy are also available on our website and in print to any stockholder who requests them. The content on our website is not incorporated by reference into this prospectus.
MANAGEMENT
The following table sets forth the names and ages of all of our directors and executive officers as of October 1, 2024. Our officers are appointed by, and serve at the pleasure of, the Board of Directors.
|
Name |
Age |
Position |
||
|
Brad Hauser |
47 |
Chief Executive Officer |
||
|
Lori Bisson |
54 |
Vice Chair and Director |
||
|
Dr. Robert Schwartz |
73 |
Chief Medical Officer |
||
|
Landy Toth |
47 |
Chief Technology Officer |
||
|
Trent Smith |
55 |
Chief Financial Officer |
||
|
Walter V. Klemp |
65 |
Executive Chairman |
||
|
Jonathan P. Foster |
60 |
Director |
||
|
David Robins |
55 |
Director |
||
|
Christopher Capelli |
64 |
Director |
Set forth below is biographical information about each of the individuals named in the table above:
Bradley Hauser, Chief Executive Officer. Mr. Hauser became our President and Chief Executive Officer effective June 17, 2024. Prior to his appointment as President and Chief Executive Officer, Mr. Hauser served as the Chief Operating Officer at Beauty Health from January 2023 to June 2024. Mr. Hauser previously served as the President and Chief Executive Officer of Soliton, Inc., a medical device company focused on developing new technology for use in aesthetics, from November 2020 to December 2021. Soliton was purchased by AbbVie in 2021 and he continued to provide transition support post-acquisition as the President and Chief Executive Officer of Soliton with Allergan Aesthetics, an AbbVie company, until July 2022. Mr. Hauser previously served as Vice President, Research and Development and General Manager for CoolSculpting at Allergan Pharmaceuticals since ZELTIQ Aesthetics, Inc. was acquired by Allergan in April 2017. Previously, he served as the Senior Vice President of Research and Development at ZELTIQ Aesthetics, Inc. from January 2017 to April 2017 and as its Vice President of Research and Development from July 2015 to January 2017. Mr. Hauser joined ZELTIQ in December 2013 as Vice President of Product and Clinical Strategy. Prior to joining ZELTIQ, he held multiple roles in the aesthetic industry, including Executive Vice President of Commercial Operations for Cutera, Director of Research and Development at Medicis and Managing Director of Product and Clinical Marketing at Solta Medical. Mr. Hauser received his B.A. degree in Human Biology from Stanford University.
Lori Bisson, Vice Chair and Director. Ms. Bisson joined us in July 2023 as a director and served as our Chief Executive Officer from July 2023 until June 2024 and as Vice Chair since June 2024. From January 2015 until June 2022, Ms. Bisson served as Chief Financial Officer of Soliton, Inc., a medical device company focused on developing new technology for use in aesthetics. Soliton was purchased by AbbVie in 2021 and she continued to provide transition support post-acquisition. Prior to joining Soliton, Ms. Bisson worked as a financial and business development consultant as a shareholder in Condon & Company, PC, from 2009 through December 2014, where she advised a number of life science companies. From 2005 to 2009, Ms. Bisson served as the Chief Financial Officer and Vice President of Operations for Zeno Corporation, a medical device company focused on new technology in the aesthetics area. Ms. Bisson previously served as the Chief Financial Officer of Gulfstream Trading, Ltd., an international oil trading organization from 2001 to 2005. From 1995 to 2001, Ms. Bisson held various positions with Drypers Corporation, a publicly traded multinational consumer products company, where she ultimately held the title of Vice President of Integrated Solutions and oversaw accounting, information technology, and logistics for the U.S. operation. Ms. Bisson began her career at Arthur Andersen, LLP as an auditor focused on consumer products companies. Ms. Bisson also serves as an advisor to Moleculin Biotech, Inc., a clinical stage pharmaceutical company focused on the development of oncology drug candidates. Ms. Bisson is a Certified Public Accountant and holds a B.A. degree in Accounting from Baylor University. We believe that Ms. Bisson’s extensive experience in the medical device field provides her with the qualifications to serve as a director.
Dr. Robert Schwartz, Chief Medical Officer. Dr. Schwartz was a co-founder of our company, has served as our Chief Medical Officer since June 2023, and served as our Chief Executive Officer from February 2022 until June 2023. Dr. Schwartz is President of the Jon DeHaan Center for Medical Innovation. He was previously Director at the Center for Applied Vascular Biology and Interventions, Mayo Foundation. Dr. Schwartz has also served as Professor and Associate Professor at the Mayo Medical School. He holds Board Certifications on the National Board of Medical Examiners, American Board of Internal Medicine, and the American Board of Internal Medicine, Cardiovascular Diseases. He has published numerous articles in peer review journals dealing with various topics in interventional cardiology. Dr. Schwartz is the recipient of various awards including the Andreas Gruentzig Award for Basic Research in Coronary Restenosis from the Thoraxcenter/European Society of Cardiology. He holds professional memberships as Fellow of the American College of Cardiology, American Heart Association, and The Society for Cardiac Angiography and Interventions. Dr. Schwartz is also a member of the Society of Atherosclerosis Imaging. Dr. Schwartz received a Master’s Degree in Electrical Engineering, and his doctorate from the University of Colorado-Denver and continued his internship, residency, and Fellowship at the Mayo Graduate School of Medicine. Dr. Schwartz has agreed to provide services to us on a part-time basis of 25% of his working time.
Landy Toth, Chief Technology Officer. Mr. Toth is a key inventor and is responsible for Autonomix’s development efforts to date. Mr. Toth founded Tricord Holdings, LLC in 2012 and Autonomix in August 2014 and has been the Chief Technology Officer since that time. In addition to these efforts, he founded and has filled the role of Chief Technology Officer of LifeLens Technologies, Inc. since September 2016. Over the past 20 years, Mr. Toth has successfully commercialized medical device technologies within startup environments. His focus has been the development and commercialization of wearable and interventional diagnostic medical technologies. He presently has 647 publications across 56 patent families. His portfolio has a grant rate greater than 70% after 2 years. Mr. Toth holds a MASc from the University of Toronto and a BASc from the University of Waterloo. Mr. Toth has also been an employee of Davos Chemical Corporation since January 2011. Mr. Toth has agreed to provide services to us on a part-time basis of 25% of his working time.
Trent Smith, Chief Financial Officer. Mr. Smith joined us in July 2023 as our Chief Financial Officer. Mr. Smith has extensive management, accounting, financial and international experience. From June 2018 to September 2022, Mr. Smith served as the Corporate Controller and Vice-President of Soliton, Inc., a medical device company focused on developing new technology for use in aesthetics that was acquired by AbbVie, Inc. in December 2021 and he continued to provide transition support post acquisition. From 2011 to 2018, Mr. Smith held various positions with InfuSystem Holdings, Inc., a national provider of infusion pumps and related services to the healthcare industry, where he served as Corporate Controller and Vice-President before a promotion to Chief Accounting Officer and Executive Vice President. From 2010 to 2011, Mr. Smith served as the Director of External Reporting of Syncreon Holdings, Inc., an international leader in global supply chain management, where he was responsible for creating the external reporting department to be compliant with SEC type reporting. From 2006 to 2010, Mr. Smith served as the Director of Accounting and Financial Reporting of Champion Homes, Inc., a leader in the manufactured housing industry and one of the largest modular homebuilders in North America. From 2005 to 2006, Mr. Smith served as the Director of External Financial Reporting for Dura Automotive Systems, a Tier 1 international designer and manufacturer of automotive components headquartered in Auburn Hills, MI. From 1999 to 2006, Mr. Smith served in various roles and divisions for Valeo, Inc., an international Tier 1 automotive supplier, including as Financial Controller and Treasurer of Valeo Distribution North America and Director of Accounting and Internal Controls for Valeo Wiper Systems, Valoe’s largest division in North America. Mr. Smith began his professional career as an auditor with Deloitte & Touche, LLP in 1995. Mr. Smith served on active duty in the United States Navy from 1987 through 1991 and the reserves until 1993. Mr. Smith is a Certified Public Accountant and a graduate of the University of Illinois where he earned a Bachelor of Science in Accounting.
Walter V. Klemp, Executive Chairman. Mr. Klemp joined us in January 2022 as our Executive Chairman. Mr. Klemp is a co-founder of Moleculin Biotech, Inc., a clinical-stage pharmaceutical company, and has served as its Chairman of the Board and Chief Executive Officer since July 2015 and as president since August 2017. From July 2018 until December 2021, Mr. Klemp served as Executive Chairman on the Board of Directors of Soliton, Inc., a medical device company focused on developing new technology for use in aesthetics. In December 2021 Soliton, Inc. was acquired by AbbVie, Inc. From November 2011 to July 2018, Mr. Klemp served as Chief Executive Officer of Soliton. Mr. Klemp served as President and Chief Executive Officer of Zeno Corporation from 2004 to April 2011, where he developed and marketed dermatology devices and drugs from concept through FDA approval and market launch. From 1987 to 2000, Mr. Klemp served as Chief Executive Officer and Chairman of Drypers Corporation, a publicly traded multinational consumer products company that was listed as #1 on the INC 500 List of America’s Fastest Growing Companies. We believe that Mr. Klemp’s extensive experience in the medical device field provide him with the qualifications to serve as a Chairman of the Board.
Jonathan P. Foster, Director. Mr. Foster joined us in January 2022 as a director. Mr. Foster has served as the Chief Financial Officer and Executive Vice President of Moleculin Biotech, Inc., a clinical-stage pharmaceutical company, since August 2016. Mr. Foster brings more than 30 years in financial experience holding a variety of executive and senior financial positions with public, private, start-up to large corporate and international companies. From February 2012 to August 2016, Mr. Foster served as Chief Financial Officer and Executive Vice President of InfuSystem Holdings, Inc., a national provider of infusion pumps and related services to the healthcare industry. From May 2011 to January 2012, Mr. Foster served as a consultant to the Chief Financial Officer of LSG Sky Chefs, USA, Inc., a subsidiary of Deutsche Lufthansa AG. Prior to that Mr. Foster served in various C-suite capacities with public and private companies with his start beginning as Manager at Deloitte & Touche, LLP. Mr. Foster served on the Board of Financial Institutions for the State of South Carolina from 2006 to 2012 and from June 2018 until December 2021 served on the Board of Directors of Soliton, Inc., a medical device company focused on developing new technology for use in aesthetics, where he was the Chairman of the Audit and Compensation Committees and the past Chairman of the Nominating & Governance Committee. In December 2021 Soliton, Inc. was acquired by AbbVie, Inc. Since June 2021, Mr. Foster has served on the Board of Directors of Volcon, Inc. where he is the past Chairman and current member of the Audit Committee, a member of the Nominating & Governance Committee and is the Chairman of the Compensation Committee. Mr. Foster is a Certified Public Accountant (South Carolina) and holds the designation of Chartered Global Management Accountant from the American Institute of Certified Public Accountants. He received his BS in Accounting from Clemson University in 1985. We believe that Mr. Foster’s extensive experience in the medical field provide him with the qualifications to serve as a director.
David Robins, Director. Mr. Robins was a co-founder of our company and has served as a director since February 2022. Mr. Robins is also a founder and board member of LifeLens that is currently developing on the body sensing devices. Since 2013, Mr. Robins has served as co-CEO of DavosPharma, a privately held corporation that focuses on supporting biotechnology and medical device companies with manufacturing their products for clinical trials and commercialization. He received his BS in Engineering Chemistry from Queen’s University in Kingston, Ontario and a MS in Chemical Engineering from Syracuse University. Mr. Robins has a significant background in taking medical devices and drugs from clinical trials to commercialization and FDA approvals. We believe that Mr. Robins’ extensive experience in medical device development provide him with the qualifications to serve as a director.
Christopher Capelli, Director. Dr. Capelli currently works at AbbVie as the Scientific Officer & Medical Device Advisor/Soliton for Allergan Aesthetics R&D Surgical Devices. Prior to being acquired by AbbVie in December 2021, he was the Vice Chairman of the Board, Chief Science Officer and Co-founder for Soliton, Inc., a medical device company that was commercializing RESONIC™ for the dermatologic esthetics marketplace based on its Rapid Acoustic Pulse (“RAP”) technology. Dr. Capelli is the lead inventor in Soliton’s RAP technology. A graduate of Massachusetts Institute of Technology (“MIT”) with a Bachelor of Science degree in Mechanical Engineering, Dr. Capelli earned his MD from the University of Wisconsin Medical School and maintains a medical license in the State of Wisconsin. As a result of his scientific work since graduating from MIT, Dr. Capelli holds over 100 issued patents and patent applications worldwide. These patents served as the basis for the creation of five companies having a total market capitalization greater than $36 billion. As a businessman, Dr. Capelli has been directly involved in the start-up of numerous venture-capital backed biomedical company ventures. We believe that Dr. Capelli’s extensive experience in medical device development provide him with the qualifications to serve as a director.
No director is related to any other director or executive officer of our company or our subsidiaries, and, there are no arrangements or understandings between a director and any other person pursuant to which such person was elected as director.
EXECUTIVE AND DIRECTOR COMPENSATION
Director Independence
The rules of the Nasdaq Stock Market, or the Nasdaq Rules, require a majority of a listed company’s board of directors to be composed of independent directors. In addition, the Nasdaq Rules require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominating and governance committees be independent. Under the Nasdaq Rules, a director will only qualify as an independent director if, in the opinion of our Board of Directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. The Nasdaq Rules also require that audit committee members satisfy independence criteria set forth in Rule 10A-3 under the Securities Exchange Act of 1934, as amended, or the Exchange Act. In order to be considered independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors, or any other board committee, accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries or otherwise be an affiliated person of the listed company or any of its subsidiaries. In considering the independence of compensation committee members, the Nasdaq Rules require that our board of directors must consider additional factors relevant to the duties of a compensation committee member, including the source of any compensation we pay to the director and any affiliations with our company.
Our board of directors undertook a review of the composition of our board of directors and its committees and the independence of each director. Based upon information requested from and provided by each director concerning his or her background, employment and affiliations, including family relationships, our board of directors has determined that each of our directors, with the exception of Ms. Bisson and Mr. Klemp, are independent as defined under the Nasdaq Rules.
Executive Officer Compensation
Summary Compensation Table – 2024
|
Name and Principal Position |
Year |
Salary |
Non-Equity Incentive Plan Compensation |
Option Awards |
All Other Compensation |
Total |
||||||||||||||||
|
Lori Bisson - Chief Executive Officer (3) |
2024 |
$ | 225,000 | $ | 150,000 | $ | 1,823,991 | $ | 17,505 | $ | 2,216,496 | |||||||||||
|
Dr. Robert Schwartz, Former Chief Executive Officer and current Chief Medical Officer |
2024 |
$ | 100,000 | $ | 35,000 | $ | 247,861 | $ | - | $ | 382,861 | |||||||||||
|
2023 |
$ | 100,000 | $ | - | $ | - | $ | - | $ | 100,000 | ||||||||||||
|
Landy Toth - Chief Technology Officer |
2024 |
$ | 187,500 | $ | - | $ | 165,241 | $ | - | $ | 352,741 | |||||||||||
|
2023 |
$ | 156,250 | $ | - | $ | - | $ | 156,250 | ||||||||||||||
|
Trent Smith - Chief Financial Officer |
2024 |
$ | 159,375 | $ | 74,250 | $ | 888,595 | $ | 18,114 | $ | 1,140,334 | |||||||||||
|
Walter Klemp - Executive Chair |
2024 |
$ | 200,000 | $ | - | $ | 165,241 | $ | - | $ | 365,241 | |||||||||||
|
2023 |
$ | 200,000 | $ | - | $ | - | $ | - | $ | 200,000 | ||||||||||||
|
(1) |
Represents the full grant date fair value of the options granted calculated in accordance with FASB ASC Topic 718. These amounts do not necessarily correspond to the actual value that may be realized by the named executive officer. Included in the award amount for Ms. Bisson and Mr. Smith were $1,468,725 and $673,783 for shares earned in fiscal 2024 awarded on June 30, 2023 and July 24, 2023, respectively. Also included in the award amount for Ms. Bisson, Dr. Schwartz, Mr. Toth, Mr. Smith and Mr. Klemp were $355,266, $247,861, $165,241, $214,812, and $165,241 for shares earned in fiscal 2024 but awarded on June 21, 2024. For a summary of the assumptions made in the valuation of the awards, please see Note 4 to our financial statements as of and for the period ended March 31, 2024 included in this prospectus.
|
|
(2) |
Other compensation amount represents health care insurance costs the Company currently pays for all employees, including $12,634 each for Ms. Bisson and Mr. Smith. In addition, the Company reimbursed Ms. Bisson $4,871 and Mr. Smith $5,480 each for COBRA reimbursements. |
|
(3) |
Ms. Bisson joined the Company on July 1, 2023. Ms. Bisson’s annual base salary was $300,000. |
|
(4) |
Mr. Smith joined the Company on July 24, 2023. Mr. Smith’s annual base salary was $225,000. |
Employment Agreements
Brad Hauser Employment Agreement
On June 17, 2024, we entered into an employment agreement with Brad Hauser pursuant to which Mr. Hauser agreed to serve as our chief executive officer and president for an initial three-year period, which may be extended on a year-to-year basis. Mr. Hauser’s agreement provides for an initial annual base salary of $450,000 (subject to an annual review and increase at the discretion of our Compensation Committee) and a target annual bonus of 60% of his base salary. Pursuant to the agreement, Mr. Hauser was granted a ten-year option (the “Inducement Options”) to purchase 45,000 shares of common stock at an exercise price equal to the closing price of our common stock on the date of the employment agreement. The option vests in four equal annual installments (or 11,250 shares each installment) on each of the succeeding four anniversary dates of the execution of the employment agreement, provided Mr. Hauser is employed by us on each vesting date. In the event of a “change of control” or the termination of the agreement by us without “cause” or by Mr. Hauser for “good reason,” all of the unvested options shall immediately vest. The Inducement Options were granted outside of our 2023 Stock Plan as an inducement material to Mr. Hauser’s entering into employment with us in accordance with Nasdaq Stock Market Listing Rule 5635(c)(4). Commencing with the year ending March 31, 2025, Mr. Hauser will be eligible to receive annual option grants as determined by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee. The number of shares underlying the target annual option grant will be equal to $1,000,000 divided by the Black-Scholes value per share of our common stock on the date of grant.
If Mr. Hauser’s employment is terminated at our election without “cause,” or by Mr. Hauser for “good reason,” Mr. Hauser shall be entitled to receive severance payments equal to twelve months of Mr. Hauser’s base salary and 100% of the target bonus for the year in which such termination occurs; provided that such amounts shall be increased by 50% if Mr. Hauser’s agreement is terminated without “cause” or by Mr. Hauser for “good reason” within three months prior to or twelve months after a “change of control.” In the event that any payments or benefits provided to Mr. Hauser would trigger the excise tax under Section 4999 of the Internal Revenue Code or any similar provision, the Company agreed to provide Mr. Hauser with a gross-up payment to ensure that, after payment of all taxes (including the excise tax, federal, state, and local income taxes, and employment taxes) imposed on the gross-up payment, Mr. Hauser receives a net amount equal to the payments or benefits Mr. Hauser would have received if the excise tax didn't apply.
Lori Bisson – Vice Chair (former Chief Executive Officer)
On June 17, 2024, we entered into an employment agreement with Lori Bisson pursuant to which Ms. Bisson agreed to serve as our Executive Vice Chair and Strategic Adviser to the Chief Executive Officer (“Vice Chair”) for a two-year period. Ms. Bisson’s agreement provides for an initial annual base salary of $150,000 (subject to an annual review and increase at the discretion of our Compensation Committee) and a target annual bonus of 50% of her base salary. Pursuant to the agreement, Ms. Bisson continued to vest in the option grants issued to Ms. Bisson in her role as chief executive officer and president in accordance with the vesting schedule set out in her initial employment agreement. In the event of a “change of control” or the termination of the agreement by us without “cause” or by Ms. Bisson for “good reason,” all of the unvested options shall immediately vest. Ms. Bisson is entitled to receive any compensation, including incentive compensation, for the fiscal year ended March 31, 2024 that has not been paid as of the date of the agreement. Commencing with the year ending March 31, 2025, Ms. Bisson will be eligible to receive annual option grants as determined by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee. Ms. Bisson agreed to waive any severance payments due to her in connection with the termination of the prior employment agreement that we entered into with her on June 30, 2023.
On June 30, 2023, we entered into an employment agreement (the “prior agreement”) with Lori Bisson pursuant to which Ms. Bisson agreed to serve as our Chief Executive Officer commencing July 1, 2023 for an initial term of three years, which would have been automatically renewed for additional one-year terms unless either party chose not to renew the prior agreement. The prior agreement provided for an initial annual base salary of $300,000. Ms. Bisson was eligible to receive an annual bonus of up to 50% of her base salary, provided that the final determination on the amount of the annual bonus, if any, would be made by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee.
Pursuant to the prior agreement, Ms. Bisson was granted a ten-year option to purchase 46,680 shares of our common stock at an exercise price of $40.00 per share. The option vests in 48 equal installments (or approximately 973 shares each installment) on each of the succeeding forty-eight monthly anniversary dates of the execution of the prior agreement, provided Ms. Bisson is either CEO or a member of the Board of Directors on such vesting date. In the event of a “change of control” or the termination of the prior agreement by us without “cause” or by Ms. Bisson for “good reason,” all of the unvested options shall immediately vest. Commencing with the first full compensation year subsequent to the completion of this offering, Ms. Bisson was eligible to receive annual option grants as determined by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee. The number of shares underlying the target annual option grant for the initial grant after the completion of the IPO was to be equal to $300,000 divided by the Black-Scholes value per share of our common stock on the date of grant. In the event Ms. Bisson’s prior agreement was terminated without “cause” or by Ms. Bisson for “good reason” within three months prior to or twelve months after of a “change of control”, all of the unvested options shall immediately vest.
If Ms. Bisson’s employment was terminated at our election without “cause”, which required 90 days advance notice, or by Ms. Bisson for “good reason,” Ms. Bisson was entitled to receive severance payments equal to twelve months of Ms. Bisson’s base salary and 100% of the target bonus for the year in which such termination occurs; provided that such amounts would have been increased by 50% if Ms. Bisson’s agreement was terminated without “cause” or by Ms. Bisson for “good reason” within three months prior to or twelve months after of a “change of control.” Ms. Bisson agreed not to compete with us until twelve months after the termination of her employment.
Trent Smith – Chief Financial Officer
On July 24, 2023, we entered into an employment agreement with Trent Smith pursuant to which Mr. Smith agreed to serve as our Chief Financial Officer for an initial term of three years, which will be automatically renewed for additional one-year terms unless either party provides 90 days written notice to the other party of its decision not to renew the agreement. The agreement provided for an initial annual base salary of $225,000. Mr. Smith is eligible to receive an annual bonus of up to 33% of his base salary, provided that the final determination on the amount of the annual bonus, if any, will be made by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee.
Pursuant to the agreement, Mr. Smith was granted a ten-year option to purchase 21,250 shares of our common stock at an exercise price of $40.00 per share. The option vests vest in four equal annual installments (or approximately 5,313 shares each installment) on each of the succeeding four anniversary dates of the execution of the employment agreement, provided Mr. Smith is CFO on such vesting date. In the event of a “change of control” or the termination of the agreement by us without “cause” or by Mr. Smith for “good reason,” all of the unvested options shall immediately vest. Commencing with the fiscal year ended March 31, 2025, Mr. Smith will be eligible to receive annual option grants as determined by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee.
If Mr. Smith’s employment is terminated upon his disability or death, at our election without “cause”, which requires 90 days advance notice, or by Mr. Smith for “good reason,” which requires 30 days advance notice, Mr. Smith shall be entitled to receive severance payments equal to nine months of Mr. Smith’s base salary and 100% of the target bonus for the year in which such termination occurs; provided that such amounts shall be increased to thirteen and one-half months of Mr. Smith’s base salary and 125% of the target bonus for the year in which such termination occurs if Mr. Smith’s agreement is terminated without “cause” or by Mr. Smith for “good reason” within three months prior to or twelve months after a “change of control.” Mr. Smith agreed not to compete with us until twelve months after the termination of his employment.
Other Agreements
In January 2022, we entered into an at-will employment letter with Dr. Schwartz to serve as our acting Chief Executive Officer on a part-time basis comprising 25% of his working time until we identified a full-time Chief Executive Officer, at which time Dr. Schwartz would become our Chief Medical Officer. Pursuant to the letter, we agreed to pay Dr. Schwartz a base salary of $100,000 per year, and to pay Dr. Schwartz an annual incentive cash bonus of up to 35% of his base salary subject to the achievement of agreed upon goals and objectives and the approved of our Board of Directors or Compensation Committee. In connection with the employment letter, we issued Dr. Schwartz a grant of 20,000 shares of our common stock. Upon the appointment of Ms. Bisson as Chief Executive Officer, Dr. Schwartz agreed to serve as our Chief Medical Officer on a part-time basis comprising 25% of his working time.
Effective January 2022, we entered into an amended and restated consulting agreement with Mr. Toth to provide services to us on a part-time basis comprising 25% of his working time. Pursuant to the consulting agreement, we agreed to pay Mr. Toth a base salary of $187,500 per year commencing in June 2022. In connection with the consulting agreement, we issued Mr. Toth a stock grant of 77,500 shares of common stock. The consulting agreement may be terminated by either party on 30 days’ notice.
In January 2022, we entered into a director offer letter with Mr. Klemp to serve as our Executive Chairman. Pursuant to the letter, we agreed to pay Mr. Klemp annual board fees of $200,000 per year. In connection with Mr. Klemp’s appointment to the Board, we issued Mr. Klemp a stock grant of 144,500 shares of common stock.
Narrative Disclosure to Summary Compensation Table
We have established for compensation purposes a compensation year that matches our fiscal year that ends March 31. Subsequent to the end of the fiscal year, our Compensation Committee completes its annual review of executive compensation and determines, after researching comparable companies, the compensation arrangements for the next compensation year.
We review compensation annually for all employees, including our executives. In setting executive base salaries and bonuses and granting equity incentive awards, we consider compensation for comparable positions in the market, the individual executive’s performance as compared to our expectations and objectives, our desire to motivate our employees to achieve short and long-term results that are in the best interests of our stockholders and a long-term commitment to our company. We do not target a specific competitive position or a specific mix of compensation among base salary, bonus or long-term incentives. Our Compensation Committee typically reviews and discusses management’s proposed compensation with the Chief Executive Officer for all executives other than the Chief Executive Officer. Based on those discussions and its discretion, the Compensation Committee then determines the compensation for each executive officer. Our Compensation Committee, without members of management present, discusses and ultimately approves the compensation of our executive officers.
Annual Base Salary
For the 2024 fiscal year, the annual base salaries for Ms. Bisson, Dr. Schwartz, Mr. Toth, Mr. Smith and Mr. Klemp were $300,000, $100,000, $187,500, $225,000 and $200,000, respectively. For the 2025 fiscal year, the base salaries for Ms. Bisson, Dr. Schwartz, Mr. Toth, Mr. Smith, Mr. Klemp and Mr. Hauser will be $375,000, $100,000, $137,500, $285,000, $150,000, and $450,000, respectively. Upon Ms. Bisson’s change in responsibilities in June 2024, her annual base salary was reduced to $150,000.
Annual Bonus and Non-Equity Incentive Plan Compensation.
We seek to motivate and reward our executives for achievements relative to our corporate goals and objectives, and with respect to their respective individual goals, for each fiscal year. For the last fiscal year, the target bonus for Ms. Bisson, Dr. Schwartz, Mr. Toth, Mr. Smith and Mr. Klemp were 50%, 35%, 0%, 33% and 0%, respectively, of their base salary. For the 2025 fiscal year, the target bonus each year for Ms. Bisson, Dr. Schwartz, Mr. Toth, Mr. Smith, Mr. Klemp and Mr. Hauser are 50%, 35%, 0%, 40%, 0%, and 60%, respectively, of their base salary.
The actual performance-based annual bonus paid is calculated by multiplying the executive’s annual base salary, target bonus percentage, the percentage attainment of the corporate goals established by the Board for such year, which represents the total potential bonus payable to our named executive officers, and the percentage attainment of the individual goals approved by our Compensation Committee with respect to our other executive officers. However, the Compensation Committee is not required to calculate bonuses in this manner and retains discretion in the amounts it awards and the factors it takes into consideration in determining bonus amounts. At the end of the fiscal year, the Compensation Committee reviews our performance against our goals and objectives and approves the extent to which we achieved each of our corporate and individual goals and objectives, and, for each named executive officer, the amount of the bonus awarded.
For the last fiscal year, bonuses were awarded based on our achievement of specified corporate goals, including a successful IPO, setting up and beginning our initial proof-of-concept trial, and searching and establishing new relationships with vendors to further the development of our sensing and ablation products, and individual goals, as applicable. Based on the level of achievement, our Compensation Committee awarded Ms. Bisson, Dr. Schwartz, Mr. Smith and Mr. Klemp 100%, respectively, of their potential bonuses for the year. These actual bonus amounts are reflected in the "Non-Equity Incentive Plan Compensation" column of the Summary Compensation Table above.
For the 2025 fiscal year, bonuses will be awarded based on our achievement of specified corporate goals, including securing strategic partnerships, progress on our pivotal trial, completion of financings, and completion of the ongoing clinical proof of concept trial. These corporate goals account for 100% of our base line bonuses. In addition, our Compensation Committee specified a series of “stretch goals,” that, if achieved, would result in an additional 20%.
Long-Term Incentives
Our 2023 Stock Plan provides for the grant of stock options, stock awards, stock unit awards and stock appreciation rights to key employees, non-employee directors and consultants.
Each year our Compensation Committee establishes a value for the expected equity grant issuable to each of our named executive officers. For options, we typically set the option exercise price and grant date fair value based on the closing price of our common stock on Nasdaq on the date of grant, however, there may be instances where we may use an average closing price, up to five days, to set the option exercise price. The shares underlying options typically vest in four equal annual installments. For other equity awards, the grant date fair value is based on the closing price of our common stock on Nasdaq on the date of grant.
For the 2025 fiscal year, the fair value of the equity grants for Ms. Bisson, Mr. Smith and Mr. Klemp were established at $300,000, $302,900, and $233,000, respectively, although the final determination for any equity grants remain at the discretion of the Compensation Committee.
Equity Awards
The following table sets forth certain information concerning our outstanding equity awards for our named executive officers at March 31, 2024.
Outstanding Equity Awards At Fiscal Year-End
|
Option Awards |
||||||||||||||||||||
|
Name and Principal Position |
Grant Date of Equity Award |
Number of Securities Underlying Unexercised Options |
Number of Securities Underlying Unexercised Options |
Option Exercise Price |
Option Expiration |
|||||||||||||||
|
Lori Bisson - Chief Executive Officer |
06/30/23 |
8,753 | 37,927 | $ | 40.00 |
06/30/33 |
||||||||||||||
|
Dr. Robert Schwartz, Former Chief Executive Officer and current Chief Medical Officer |
- | - | - | $ | - | - | ||||||||||||||
|
Trent Smith - Chief Financial Officer |
07/24/23 |
- | 21,250 | $ | 40.00 |
07/24/33 |
||||||||||||||
|
Landy Toth - Chief Technology Officer |
- | - | - | $ | - | - | ||||||||||||||
|
Walter Klemp - Executive Chairman |
- | - | - | $ | - | - | ||||||||||||||
|
(1) |
The shares underlying the options granted on June 30, 2023 for Ms. Bisson vest in equal monthly installments over a four-year period (i.e., 1/48th of each grant vests each month on the anniversary of the grant date). The shares underlying the options granted on July 24, 2023 for Mr. Smith vest in annual installments over a four-year period (i.e., one-quarter of each grant vests on the first, second, third and fourth anniversary of the grant date), subject to continued service with us through each applicable vesting date. |
Director Compensation
In January 2022, we entered into a director offer letter with Mr. Klemp to serve as our Executive Chairman. Pursuant to the letter, we agreed to pay Mr. Klemp annual board fees of $200,000 per year commencing April 1, 2022. In connection with Mr. Klemp’s appointment to the Board, we issued Mr. Klemp a stock grant of 144,500 shares of common stock.
In January 2022, we entered into a director offer letter with Mr. Foster to serve as a director. Pursuant to the letter, we agreed to pay Mr. Foster annual board fees of $50,000 per year. In connection with Mr. Foster’s appointment to the Board, we issued Mr. Foster a stock grant of 35,000 shares of common stock.
In February 2022, we entered into a director offer letter with Mr. Robins to serve as a director. In connection with Mr. Robins’ appointment to the Board, we issued Mr. Robins a stock grant of 5,000 shares of common stock.
In September 2023, we entered into a director offer letter with Mr. Capelli to serve as a director. In connection with Mr. Capelli’s appointment to the Board, we issued Mr. Capelli a stock options to purchase up to 3,750 shares of common stock.
Commencing upon the closing of our IPO in January 2024, our non-employee directors began to receive annual compensation of $50,000.
In May 2024, the Board of Directors approved an updated non-employee director compensation plan, pursuant to which upon the initial appointment (or election) of an non-employee director to the Board, the non-employee director shall be issued a 10-year option to purchase 3,750 shares of the Company’s common stock, under the 2023 Stock Plan, that will vest in three equal annual installments over a three-year period. In addition, on the date of our annual meeting, each non-employee director that is re-elected at the Annual Shareholder Meeting will be issued a 10-year option to purchase 2,500 shares of the Company’s common stock, under the 2023 Stock Plan that will vest quarterly over a one-year period. In addition, each non-employee director will receive an annual compensation of $40,000 and special service pay amounts based on service for committee responsibilities as follows: Audit Committee Chair - $15,000; Compensation Committee Chair - $10,000; and Nominating and Governance Chair - $7,500. Each non-chair committee member will also received the following compensations: Audit Committee member - $7,500; Compensation Committee member - $5,000; and Nominating and Governance Committee member - $3,750.
The following table sets forth the total compensation earned by our non-employee directors in fiscal 2024. Mr. Klemp’s compensation is fully reflected in the “Summary Compensation Table” above:
| Name | Year |
Fees earned or paid in cash ($) |
Option awards ($)(1) |
Total ($) |
||||||||||
| Jonathan P. Foster | 2024 | $ | 50,000 | (2) | $ | - | $ | 50,000 | ||||||
| David Robins | 2024 | $ | 8,333 | (3) | $ | - | $ | 8,333 | ||||||
| Christopher Capelli | 2024 | $ | 8,333 | (3) | $ | 116,980 | $ | 125,313 | ||||||
|
(1) |
Represents the full grant date fair value of the option award our board approved and granted to each non-employee director, calculated in accordance with FASB ASC Topic 718. These amounts do not necessarily correspond to the actual value that may be realized by the director. For a summary of the assumptions made in the valuation of the awards, please see Note 4 to our audited financial statements as of and for the period ended March 31, 2024 included in this prospectus. As of March 31, 2024, the aggregate number of shares outstanding under all options to purchase our common stock held by our non-employee directors were: Mr. Capelli – 3,750 shares. |
|
(2) |
Annual cash Board fee commenced with agreement dated April 1, 2022. |
|
(3) |
Annual cash Board fee commenced with the completion of the Company’s IPO on January 29, 2024. |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
In addition to the compensation arrangements, including employment, termination of employment and change in control arrangements, with our directors and executive officers, including those discussed in the sections titled “Management” and “Executive and Director Compensation,” the following is a description of each transaction since January 1, 2022 or any currently proposed transaction in which:
• we have been or are to be a party to;
• the amount involved exceeded or exceeds $120,000 or 1% of the average of our total assets as of the end of the last two completed fiscal years; and
• any of our directors, executive officers or holders of more than 5% of our outstanding capital stock, or any immediate family member of, or person sharing the household with, any of these individuals or entities, had or will have a direct or indirect material interest.
We utilize a consulting firm that is owned by Matthew Lourie, our former Chief Financial Officer, to provide accounting and financial reporting services and to pay certain expenses on behalf of the Company. During the years ended March 31, 2024 and 2023, we incurred fees of $59,450 and $49,056 for these services, respectively, excluding officer compensation. During the three months ended June 30, 2024 and 2023, the Company incurred fees of $0 and $23,063, respectively, for these services, excluding officer compensation. As of June 30, 2024 and March 31, 2024, we owed this consulting firm $0 and $609, respectively, for services and expenses.
As part of the March 2022 sale of our common stock for cash, a member of the Board of Directors purchased 2,500 shares of common stock for $100,000 of cash proceeds. As part of the March 2023 sale of common stock for cash, three members of the Board of Directors purchased, in the aggregate, 8,125 shares of common stock for $325,000 of cash proceeds.
In December 2021, we granted a company affiliated with certain early investors in the Company a license to our technology for use in the field of cardiology. In July 2023, we entered into a termination agreement for the license agreement in exchange for the issuance, upon the closing of our IPO, of a warrant to purchase 80,000 shares of our common stock at an exercise price of $0.02 per share. The shares underlying the warrant are subject to a lockup agreement for a period of six months after the closing of the IPO with respect to 12.5% of the shares and twelve months after the closing of the IPO for the remainder of the shares. We agreed to provide demand registration rights to the licensee with respect to the resale of the shares of common stock underlying the warrants. One of our directors, David Robins, holds a 19.5% interest in the company receiving the warrant.
During the year ended March 31, 2022, in connection with the sale of securities and the entrance of the license agreement discussed above, we paid a total of $175,000 to the licensee, which was recorded as general and administrative expense, and incurred an additional $26,282 of legal costs from the licensee related to the license, which were paid during the year ended March 31, 2023.
In September 2023, we commenced a private placement for up to $2.0 million in principal amount of convertible notes with a maturity date of December 31, 2025. For each dollar in principal amount of convertible notes purchased, we issued a warrant to purchase 0.0125 shares of our common stock, with an exercise price of $20.00 per share. On the closing of our IPO, the principal amount of the convertible notes converted into our common stock at a conversion price of $40.00 per share. Subsequent to September 30, 2023, members of the Company’s management, Board of Directors and an immediate family member of the Company’s management, collectively purchased $500,000 ($400,000 (for management and members of the Board of Directors) and $100,000 (for the immediate family member of a member of the Company’s management, respectively) in principal amount of convertible notes in the private placement.
Policies and Procedures for Related Party Transactions
Our audit committee charter provides that our audit committee is responsible for reviewing and approving in advance any related party transaction. This will cover, with certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships in which we were or will be a participant to, where the amount involved exceeds the lesser of $120,000 or one percent of the average of our total assets at year-end for the last two completed fiscal years, and a related person had or will have a direct or indirect material interest, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. In determining whether to approve a proposed transaction, our audit committee will consider all relevant facts and circumstances including: (i) the materiality and character of the related party’s direct or indirect interest; (ii) the commercial reasonableness of the terms; (iii) the benefit or perceived benefit, or lack thereof, to us; (iv) the opportunity cost of alternate transactions; and (v) the actual or apparent conflict of interest of the related party.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth information, as of October 1, 2024, regarding beneficial ownership of our common stock by:
• each of our directors;
• each of our named executive officers;
• all directors and current executive officers as a group; and
• each person, or group of affiliated persons, known by us to beneficially own more than five percent of our shares of common stock.
The percentage of beneficial ownership before the offering is based on 1,152,120 shares outstanding as of October 1, 2024. Beneficial ownership is determined according to the rules of the SEC, and generally means that person has beneficial ownership of a security if he or she possesses sole or shared voting or investment power of that security and includes options that are currently exercisable or exercisable within 60 days. Each director or officer, as the case may be, has furnished us with information with respect to beneficial ownership. Except as otherwise indicated, we believe that the beneficial owners of common stock listed below, based on the information each of them has given to us, have sole investment and voting power with respect to their shares, except where community property laws may apply. Except as otherwise noted below, the address for each person or entity listed in the table is c/o Autonomix Medical, Inc., 21 Waterway Avenue, Suite 300, The Woodlands, TX 77380.
|
Shares beneficially owned |
Percent of Class |
|||||||
| Name of Beneficial Owner | ||||||||
| Executive officers and directors: | ||||||||
| Lori Bisson | 20,283 | (1) | 1.7 | % | ||||
| Dr. Robert Schwartz | 35,764 | 3.1 | % | |||||
| Landy Toth | 101,314 | 8.8 | % | |||||
| Walter Klemp | 150,750 | 13.1 | % | |||||
| Jonathan P. Foster | 36,563 | 3.2 | % | |||||
| Christopher Capelli | 3,750 | (1) | * | |||||
| David Robins | 26,581 | 2.3 | % | |||||
| Trent Smith | 9,063 | * | ||||||
| Brad Hauser | - | - | ||||||
| All current Executive Officers and Directors as a group (9 persons) | 384,068 | 32.5 | % | |||||
| 5% Shareholders: | ||||||||
| BioStar Ventures III, L.P. | 120,816 | (3) | 10.5 | % | ||||
* Less than 1%
(1) Consists of shares of common stock underlying options and convertible notes to purchase common stock that vest within 60 days of October 1, 2024.
(2) Includes a portion of 80,000 warrants to purchase shares of the Company’s common stock that was held by Impulse Medical, Inc., in which Mr. Robins owns 19.5% through Portsmouth Therapeutics, Inc., an entity in which Mr. Robins also owns a 33.33% interest. The shares underlying the warrants are subject to a lockup agreement for a period of six months after the closing of the Company’s IPO with respect to 12.5% of the shares issued and twelve months after the closing of the Company’s IPO for the remainder of the shares.
(3) The business address of BioStar Ventures III, L.P. is 206, Bridge Street, Charlevoix, MI 49720. General Partner, BioStar Ventures III, LLC, 206, Bridge Street, Charlevoix, MI 49720 has voting and dispositive power over the shares held by BioStar Ventures III, L.P. Louis A. Cannon serves as the Senior Managing Director of BioStar Ventures III, LLC.
DESCRIPTION OF CAPITAL STOCK
The following summary of the rights of our capital stock is not complete and is subject to and qualified in its entirety by reference to our certificate of incorporation and bylaws, copies of which are filed as exhibits to the registration statement of which this prospectus forms a part, which are incorporated by reference herein, and the applicable provisions of the Delaware General Corporation Law.
Common Stock
We are authorized to issue up to 500,000,000 shares of common stock. Shares of our common stock have the following rights, preferences and privileges:
Voting
Each holder of common stock is entitled to one vote for each share of common stock held on all matters submitted to a vote of stockholders. Any action at a meeting at which a quorum is present will be decided by a majority in voting power of the votes cast (excluding abstentions and broker non-votes), except in the case of any election of directors, which will be decided by a plurality of votes cast. There is no cumulative voting.
Dividends
Holders of our common stock are entitled to receive dividends when, as and if declared by our board of directors out of funds legally available for payment, subject to the rights of holders, if any, of any class of stock having preference over the common stock. Any decision to pay dividends on our common stock will be at the discretion of our board of directors. Our board of directors may or may not determine to declare dividends in the future. The board’s determination to issue dividends will depend upon our profitability and financial condition any contractual restrictions, restrictions imposed by applicable law and the SEC, and other factors that our board of directors deems relevant.
Liquidation Rights
In the event of a voluntary or involuntary liquidation, dissolution or winding up of the company, the holders of our common stock will be entitled to share ratably on the basis of the number of shares held in any of the assets available for distribution after we have paid in full, or provided for payment of, all of our debts and after the holders of all outstanding series of any class of stock have preference over the common stock, if any, have received their liquidation preferences in full.
Number of Holders
There are approximately 4,800 holders of our common stock as of October 1, 2024.
Other
Our issued and outstanding shares of common stock are fully paid and nonassessable. Holders of shares of our common stock are not entitled to preemptive rights. Shares of our common stock are not convertible into shares of any other class of capital stock, nor are they subject to any redemption or sinking fund provisions.
Preferred Stock
We are authorized to issue up to 10,000,000 shares of preferred stock. Our certificate of incorporation authorizes the board to issue these shares in one or more series, to determine the designations and the powers, preferences and relative, participating, optional or other special rights and the qualifications, limitations and restrictions thereof, including the dividend rights, conversion or exchange rights, voting rights (including the number of votes per share), redemption rights and terms, liquidation preferences, sinking fund provisions and the number of shares constituting the series. Our board of directors could, without stockholder approval, issue preferred stock with voting and other rights that could adversely affect the voting power and other rights of the holders of common stock and which could have the effect of making it more difficult for a third party to acquire, or of discouraging a third party from attempting to acquire, a majority of our outstanding voting stock.
Convertible Notes Payable
On September 9, 2023, our Board of Directors authorized an offering up to $2.0 million in unsecured, non-interest bearing convertible promissory notes (the “Notes”) and accompanying warrants (the “Bridge Financing Warrants”) (collectively, the “Bridge Offering”) that will mature on December 31, 2025. The Notes provide that, on the closing date of the IPO, the outstanding principal would be automatically converted into common stock at the conversion price of $40.00, provided that certain holders of the Notes are not permitted to convert such Notes to the extent that the holders or any of its affiliates would beneficially own in excess of 4.99% (the “Beneficial Ownership Limit”) of our common stock after such conversion. Each dollar in principal amount of the convertible notes purchased were accompanied by a five-year Bridge Financing Warrant to purchase approximately 0.0125 shares of common stock with an exercise price of $20.00 per share as discussed in “Warrants – Pre-IPO Warrants” below.
As of June 30, 2024, due to the Beneficial Ownership Limit, we have outstanding Notes in the aggregate amount of $1,330,000 that convert into 33,250 shares of our common stock.
Warrants
License Termination Warrants
As of the date hereof, we have outstanding warrants to purchase 80,000 shares of our common stock at an exercise price of $0.02 per share expiring January 2029. The shares underlying the warrant are subject to a lockup agreement for a period of six months after the closing of our IPO with respect to 12.5% of the shares issued and twelve months after the closing of our IPO for the remainder of the shares. We agreed to provide demand registration rights to the licensee with respect to the resale of the shares of common stock underlying the warrants.
Pre-IPO Warrants
As of October 1, 2024, we have outstanding warrants to purchase 4,542 shares of our common stock at a weighted average exercise price of $11.99 per share expiring through March 2032 and Bridge Financing Warrants to purchase an aggregate of 25,003 shares of our common stock at an exercise price of $20.00 per share expiring through October 2028. The foregoing warrants provide that no holder of these warrants will be permitted to exercise such warrants to the extent that the holder or any of its affiliates would beneficially own in excess of 4.99% of our common stock after such exercise.
Selling Agent Warrants
In connection with our IPO, we issued approximately 2,989 shares of our common stock issuable upon exercise of warrants issued to the selling agent in our IPO (the “Selling Agent’s Warrants”). The Selling Agent’s Warrants are exercisable commencing six months after the date of commencement of sales in our IPO and will be exercisable until the fifth anniversary of such date. The exercise price for the Selling Agent’s Warrants is $125.00 per share. The Selling Agent’s Warrants are not redeemable.
Limitations on Liability and Indemnification of Officers and Directors
Our certificate of incorporation and bylaws limit the liability of our officers and directors and provide that we will indemnify our officers and directors, in each case, to the fullest extent permitted by the Delaware General Corporation Law.
We have entered into separate indemnification agreements with each of our directors and executive officers. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors or executive officers, we have been informed that in the opinion of the Commission such indemnification is against public policy and is therefore unenforceable.
Certificate of Incorporation and Bylaw Provisions
Our certificate of incorporation and bylaws include a number of anti-takeover provisions that may have the effect of encouraging persons considering unsolicited tender offers or other unilateral takeover proposals to negotiate with our board of directors rather than pursue non-negotiated takeover attempts. These provisions include:
Advance Notice Requirements. Our bylaws establish advance notice procedures with regard to stockholder proposals relating to the nomination of candidates for election as directors or new business to be brought before meetings of stockholders. These procedures provide that notice of stockholder proposals must be timely and given in writing to our corporate Secretary. Generally, to be timely, notice must be received at our principal executive offices not less than 90 days nor more than 120 days prior to the one-year anniversary of the preceding year’s annual meeting. The notice must contain the information required by the bylaws, including information regarding the proposal and the proponent.
Special Meetings of Stockholders. Our certificate of incorporation provides that special meetings of stockholders may be called at any time by, or at the direction of, only the Chairman of the Board, the Chief Executive Officer, the President or the board of directors.
No Written Consent of Stockholders. Our certificate of incorporation provides that any action required or permitted to be taken by stockholders must be effected at a duly called annual or special meeting of stockholders and may not be effected by any consent in writing by such stockholders.
Exclusive Forum Provision. Our certificate of incorporation provides that the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of a fiduciary duty owed by any of our directors or officers to us or our stockholders, (iii) any action asserting a claim against us arising pursuant to any provision of the Delaware General Corporation Law (the “DGCL”), or our certificate of incorporation or the bylaws, and (iv) any action asserting a claim against us governed by the internal affairs doctrine. This provision would not apply to suits brought to enforce a duty or liability created by the Exchange Act or Securities Act.
This choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and employees. In addition, this forum selection provision may impose additional litigation costs on stockholders in pursuing the claims identified above, particularly if the stockholders do not reside in or near the State of Delaware. Alternatively, a court could find these provisions of our certificate of incorporation to be inapplicable or unenforceable in respect of one or more of the specified types of actions or proceedings, which may require us to incur additional costs associated with resolving such matters in other jurisdictions, which could adversely affect our business and financial condition.
Amendment of Bylaws. Our stockholders may amend any provisions of our bylaws by obtaining the affirmative vote of the holders of at least a majority of the voting power of all the then-outstanding shares of our voting stock with the power to vote generally in an election of directors, voting together as a single class.
Preferred Stock. Our certificate of incorporation authorizes our board of directors to create and issue rights entitling our stockholders to purchase shares of our stock or other securities. The ability of our board to establish the rights and issue substantial amounts of preferred stock without the need for stockholder approval may delay or deter a change in control of us. See “Preferred Stock” above.
Delaware Takeover Statute
We are subject to Section 203 of the DGCL which, subject to certain exceptions, prohibits a Delaware corporation from engaging in any “business combination” (as defined below) with any interested stockholder for a period of three years following the date that such stockholder became an interested stockholder, unless: (1) prior to such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; (2) on consummation of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding those shares owned (a) by persons who are directors and also officers and (b) by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to this plan will be tendered in a tender or exchange offer; or (3) on or subsequent to such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2⁄3% of the outstanding voting stock that is not owned by the interested stockholder.
Section 203 of the DGCL defines generally “business combination” to include: (1) any merger or consolidation involving the corporation and the interested stockholder; (2) any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; (3) subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; (4) any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; or (5) the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. In general, Section 203 defines an “interested stockholder” as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by such entity or person.
Transfer Agent
The transfer agent for our common stock is Equity Stock Transfer, LLC.
DESCRIPTION OF SECURITIES WE ARE OFFERING
Units
We are offering up to 698,812 Common Stock Units, with each Common Stock Unit consisting of: (i) one share of our common stock and (ii) a Series A Warrant to purchase one share of our common stock.
We are also offering to each purchaser whose purchase of Common Stock Units in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase, if the purchaser so chooses, PFW Units in lieu of Common Stock Units that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock. Each PFW Unit consists of: (i) one Pre-Funded Warrant and (ii) a Series A Warrant to purchase one share of our common stock. The Common Warrants included in the PFW Units are identical to the Common Warrants included in the Common Stock Units.
The shares of common stock in the Common Stock Units or the Pre-Funded Warrants in the PFW Units, as applicable, and the accompanying Common Warrants, can only be purchased together in this offering but will be issued separately and will be immediately separable upon issuance.
Pre-Funded Warrants
The following summary of certain terms and provisions of Pre-Funded Warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the Pre-Funded Warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part.
Form. The Pre-Funded Warrants will be issued as individual warrant agreements to the investors. You should review the form of Pre-Funded Warrant, filed as an exhibit to the registration statement of which this prospectus forms a part, for a complete description of the terms and conditions applicable to the Pre-Funded Warrants.
Exercisability. The Pre-Funded Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full in immediately available funds for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as described below). A holder (together with its affiliates) may not exercise any portion of the Pre-Funded Warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder, 9.99%) of the outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s Pre-Funded Warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Pre-Funded Warrants. Purchasers of Pre-Funded Warrants in this offering may also elect prior to the issuance of the Pre-Funded Warrants to have the initial exercise limitation set at 9.99% of our outstanding common stock. No fractional shares of common stock will be issued in connection with the exercise of a Pre-Funded Warrant. In lieu of fractional shares, we will pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price.
Duration and Exercise Price. The exercise price per whole share of our common stock purchasable upon the exercise of the Pre-Funded Warrants is $0.001 per share of common stock. The Pre-Funded Warrants will be immediately exercisable and may be exercised at any time until the Pre-Funded Warrants are exercised in full. The exercise price of the Pre-Funded Warrants is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common stock and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Cashless Exercise. In lieu of making the cash payment otherwise contemplated to be made to us upon the exercise of any Pre-Funded Warrants in payment of the aggregate exercise price, the holder may, at its option, instead receive upon such exercise (either in whole or in part) only the net number of shares of common stock determined according to a formula set forth in the Pre-Funded Warrants. Notwithstanding anything to the contrary, there are no circumstances that would require us to make any cash payments or net cash settle the Pre-Funded Warrants to the holders.
Transferability. Subject to applicable laws, the Pre-Funded Warrants may be offered for sale, sold, transferred or assigned at the option of the holder upon surrender of the Pre-Funded Warrant to us together with the appropriate instruments of transfer.
Exchange Listing. We do not plan on applying to list the Pre-Funded Warrants on Nasdaq, any other national securities exchange or any other nationally recognized trading system.
Fundamental Transactions. In the event of a fundamental transaction, as described in the Pre-Funded Warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the Pre-Funded Warrants will be entitled to receive upon exercise of the Pre-Funded Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Pre-Funded Warrants immediately prior to such fundamental transaction.
Rights as a Stockholder. Except by virtue of such holder’s ownership of shares of our common stock, the holder of a Pre-Funded Warrant does not have the rights or privileges of a holder of our common stock, including any voting rights, until the holder exercises the Pre-Funded Warrant.
Series A Warrants
The following summary of certain terms and provisions of Series A Warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the Series A Warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part.
Duration and Exercise Price. Each Series A Warrant offered hereby has an assumed initial exercise price per share equal to $____ (representing ____), subject to adjustment. The Series A Warrants will be immediately exercisable. The exercise price and number of shares of common stock issuable upon exercise for each Series A Warrant is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our shares of common stock and the exercise price. Equity Stock Transfer, LLC has agreed to serve as warrant agent with respect to the Series A Warrants pursuant to a warrant agency agreement to be entered into concurrently with the closing of this offering. The Series A Warrants will expire five years from the closing date of this offering.
Exercisability. The Series A Warrants will be exercisable, at the option of the holder, in whole or in part, by delivering to us a duly-executed exercise notice, with payment in full for the number of shares of common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below) due prior to issuance of the common stock. A holder (together with its affiliates) may not exercise any portion of the Series A Warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder, prior to issuance, 9.99%) of the outstanding shares of common stock immediately after exercise, referred to herein as the beneficial ownership limitation. However, upon notice from the holder to us, the holder may decrease or increase the beneficial ownership limitation, which may not exceed 9.99% of the number of shares of common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Series A Warrants, provided that any increase in the beneficial ownership limitation will not take effect until 61 days following notice to us. No fractional shares of common stock will be issued in connection with the exercise of a Series A Warrant. In lieu of fractional shares, we will either pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price or round up to the next whole share.
Cashless Exercise. At any time, in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of the shares of common stock determined according to a formula set forth in the warrants.
Transferability. Subject to applicable laws, a Series A Warrant may be transferred at the option of the holder upon surrender of the Series A Warrant to us together with the appropriate instruments of transfer.
Exchange Listing. There is no trading market available for the Series A Warrants on any securities exchange or nationally recognized trading system. We do not intend to list the Series A Warrants on any securities exchange or nationally recognized trading system.
Right as a Stockholder. Except as otherwise provided in the Series A Warrants, including as described below under “Pro Rata Distributions,” or by virtue of such holder’s ownership of our shares of common stock, the holders of the Series A Warrants do not have the rights or privileges of holders of our shares of common stock, including any voting rights, until they exercise their Series A Warrants.
Subsequent Rights Offerings. In addition to in the event of stock dividends, stock splits, reorganizations or similar events affecting our shares of common stock and the exercise price, if at any time the Company grants, issues or sells any specified common stock equivalents or rights to purchase stock, warrants, securities or other property pro rata to the record holders of any class of shares of Common Stock (the “Purchase Rights”), then the holder of a Series A Warrant will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the holder could have acquired if the holder had held the number of shares of common stock acquirable upon complete exercise of the holder’s Series A Warrant, without regard to any limitations on exercise hereof, including without limitation, the beneficial ownership limitation, immediately before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the date as of which the record holders of shares of common stock are to be determined for the grant, issue or sale of such Purchase Rights (provided, however, that, to the extent that the holder’s right to participate in any such Purchase Right would result in the holder exceeding the beneficial ownership limitation, then the holder shall not be entitled to participate in such Purchase Right to such extent (or beneficial ownership of such shares of common stock as a result of such Purchase Right to such extent) and such Purchase Right to such extent shall be held in abeyance for the holder until such time, if ever, as its right thereto would not result in the holder exceeding the beneficial ownership limitation).
Pro Rata Distributions. During such time as a Series A Warrant is outstanding and has not reached its termination date, if the Company shall declare or make any dividend or other distribution of its assets (or rights to acquire its assets) to holders of shares of common stock, by way of return of capital or otherwise (including, without limitation, any distribution of cash, stock or other securities, property or options by way of a dividend, spin off, reclassification, corporate rearrangement, scheme of arrangement or other similar transaction) (a “Distribution”), at any time after the issuance of the Series A Warrants, then, in each such case, the holder of a Series A Warrant shall be entitled to participate in such Distribution to the same extent that the holder would have participated therein if the holder had held the number of shares of common stock acquirable upon complete exercise of the Series A Warrant (without regard to any limitations on exercise hereof, including without limitation, the beneficial ownership limitation) immediately before the date of which a record is taken for such Distribution, or, if no such record is taken, the date as of which the record holders of shares of common stock are to be determined for the participation in such Distribution (provided, however, that, to the extent that the holder’s right to participate in any such Distribution would result in the holder exceeding the beneficial ownership limitation, then the holder shall not be entitled to participate in such Distribution to such extent (or in the beneficial ownership of any shares of common stock as a result of such Distribution to such extent) and the portion of such Distribution shall be held in abeyance for the benefit of the holder until such time, if ever, as its right thereto would not result in such Holder exceeding the beneficial ownership limitation).
Fundamental Transaction. In the event of a fundamental transaction, as described in the Series A Warrants and generally including any reorganization, recapitalization or reclassification of our shares of common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding shares of common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding shares of common stock, the holders of the Series A Warrants will be entitled to receive upon exercise of the Series A Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Series A Warrants immediately prior to such fundamental transaction.
Change in Control. In the event of a Change in Control, as defined in the Series A Warrants, which generally includes any Fundamental Transaction other than any reorganization, recapitalization or reclassification of the Common Stock in which holders of the Company’s voting power immediately prior to such event continue after such event to be the holders of the voting power of the surviving entity, (ii) pursuant to a migratory merger effected solely for the purpose of changing the jurisdiction of incorporation of the Company or (iii) a merger in connection with a bona fide acquisition by the Company of any person in which (x) the gross consideration paid, directly or indirectly, by the Company in such acquisition is not greater than 50% of the Company’s market capitalization as calculated on the date of the consummation of such merger and (y) such merger does not contemplate a change to the identity of a majority of the board of directors of the Company, warrant holders may require the company to purchase the remaining unexercised portion of a Series A Warrant for an amount equal to the Black-Scholes Value (as defined in the relevant warrant) of that portion, as of the date of the Change of Control, unless the Change of Control is not within the Company’s control, as described in the warrant. In that event, holders will instead be entitled to receive the same type or form of consideration (and in the same proportion), at the Black-Scholes Value of the unexercised portion of the warrant, that is being offered and paid to the common shareholders.
Global Warrants. Series A Warrants shall be issued in book entry form (the “Global Warrants”). All of the Series A Warrants shall initially be represented by one or more Global Warrants deposited with the warrant agent and registered in the name of Cede & Co., a nominee of The Depository Trust Company (the “Depositary”), or as otherwise directed by the Depositary. Ownership of beneficial interests in the Series A Warrants shall be shown on, and the transfer of such ownership shall be effected through, records maintained by (i) the Depositary or its nominee for each Global Warrant or (ii) institutions that have accounts with the Depositary (such institution, with respect to a Series A Warrant in its account, a “Participant”). If the Depositary subsequently ceases to make its book-entry settlement system available for the Series A Warrants, the Company may instruct the warrant agent regarding other arrangements for book-entry settlement. In the event that the Series A Warrants are not eligible for, or it is no longer necessary to have the Series A Warrants available in, book-entry form, the warrant agent shall provide written instructions to the Depositary to deliver to the warrant agent for cancellation each Global Warrant, and the Company shall instruct the warrant agent to deliver to each holder of a Series A Warrant a Common Warrant certificate. A holder of a Series A Warrant has the right to elect at any time or from time to time a warrant exchange (as defined in the warrant agency agreement). Upon written notice by a holder to the warrant agent for the exchange of some or all of such holder’s Global Warrants for a Series A Warrant certificate evidencing the same number of Series A Warrants, which request shall be in the form specified in the warrant agency agreement, the warrant agent shall promptly effect the warrant exchange and shall promptly issue and deliver to the holder a warrant certificate for such number of Series A Warrants in the name set forth in the warrant certificate request notice. In the event a beneficial owner requests a warrant exchange, upon issuance of the paper warrant certificate, the Company shall act as warrant agent and the terms of the paper warrant certificate so issued shall exclusively govern in respect thereof.
MATERIAL UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following discussion is a summary of the material U.S. federal income tax consequences of the acquisition, ownership and disposition of the common stock, Pre-Funded Warrants and Common Warrants acquired in this offering and does not purport to be a complete analysis of all the potential tax considerations relating thereto. This discussion is based on the current provisions of the Internal Revenue Code of 1986, as amended, referred to as the Code, existing and proposed U.S. Treasury regulations promulgated thereunder, and administrative rulings and court decisions in effect as of the date hereof, all of which are subject to change at any time, possibly with retroactive effect, with the resulting U.S. federal income tax consequences being different from those set forth below. No ruling has been or will be sought from the Internal Revenue Service, or IRS, with respect to the matters discussed below, and there can be no assurance the IRS will not take a contrary position regarding the tax consequences of the acquisition, ownership or disposition of the common stock, Pre-Funded Warrants or Common Warrants, or that any such contrary position would not be sustained by a court.
We assume in this discussion that the shares of common stock, Pre-Funded Warrants and Common Warrants will be held as capital assets (generally, property held for investment). This discussion does not address all aspects of U.S. federal income taxes, does not discuss the potential application of the Medicare contribution tax or the alternative minimum tax and does not address state or local taxes or U.S. federal gift and estate tax laws, except as specifically provided below with respect to non-U.S. holders, or any non-U.S. tax consequences that may be relevant to holders in light of their particular circumstances. This discussion also does not address the special tax rules applicable to particular holders, such as:
|
● |
persons who acquired our common stock, Pre-Funded Warrants or Common Warrants as compensation for services; |
|
● |
traders in securities that elect to use a mark-to-market method of accounting for their securities holdings; |
|
● |
persons that own, or are deemed to own, more than 5% of our common stock (except to the extent specifically set forth below); |
|
● |
persons required for U.S. federal income tax purposes to conform the timing of income accruals to their financial statements under Section 451(b) of the Code (except to the extent specifically set forth below); |
|
● |
persons for whom our common stock constitutes “qualified small business stock” within the meaning of Section 1202 of the Code or “Section 1244 stock” for purposes of Section 1244 of the Code; |
|
● |
persons deemed to sell our common stock, Pre-Funded Warrants or Common Warrants under the constructive sale provisions of the Code; |
|
● |
banks or other financial institutions; |
|
● |
brokers or dealers in securities or currencies; |
|
● |
tax-exempt organizations or tax-qualified retirement plans; |
|
● |
pension plans; |
|
● |
regulated investment companies or real estate investment trusts; |
|
● |
persons that hold the common stock, Pre-Funded Warrants or Common Warrants as part of a straddle, hedge, conversion transaction, synthetic security or other integrated investment; |
|
● |
insurance companies; |
|
● |
controlled foreign corporations, passive foreign investment companies, or corporations that accumulate earnings to avoid U.S. federal income tax; and |
|
● |
certain U.S. expatriates, former citizens, or long-term residents of the United States. |
In addition, this discussion does not address the tax treatment of partnerships (including any entity or arrangement classified as a partnership for U.S. federal income tax purposes) or other pass-through entities or persons who hold shares of common stock, Pre-Funded Warrants or Common Warrants through such partnerships or other entities which are pass-through entities for U.S. federal income tax purposes. If such a partnership or other pass-through entity holds shares of common stock, Pre-Funded Warrants or Common Warrants, the treatment of a partner in such partnership or investor in such other pass-through entity generally will depend on the status of the partner or investor and upon the activities of the partnership or other pass-through entity. A partnership or other pass-through entity, a partner in such a partnership and an investor in such other pass-through entity that will hold shares of common stock, Pre-Funded Warrants or Common Warrants should consult his, her or its own tax advisor regarding the tax consequences of the ownership and disposition of shares of common stock, Pre-Funded Warrants or Common Warrants.
This discussion of U.S. federal income tax considerations is for general information purposes only and is not tax advice. Prospective investors should consult their own tax advisors regarding the U.S. federal, state, local and non-U.S. income and other tax considerations of acquiring, holding and disposing of our common stock, Pre-Funded Warrants and Common Warrants.
For the purposes of this discussion, a “U.S. Holder” means a beneficial owner of shares of common stock, Pre-Funded Warrants or Common Warrants that is for U.S. federal income tax purposes (a) an individual citizen or resident of the United States (b) a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes), created or organized in or under the laws of the United States, any state thereof or the District of Columbia, (c) an estate the income of which is subject to U.S. federal income taxation regardless of its source, or (d) a trust if it (1) is subject to the primary supervision of a court within the United States and one or more U.S. persons (within the meaning of Section 7701(a)(30) of the Code) has the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a domestic trust. A “Non-U.S. Holder” is, for U.S. federal income tax purposes, a beneficial owner of shares of common stock, Pre-Funded Warrants or Common Warrants that is not a U.S. Holder or a partnership (or other entity treated as a partnership) for U.S. federal income tax purposes.
Treatment of Pre-Funded Warrants
Although it is not entirely free from doubt, we believe that a Pre-Funded Warrant should be treated as a share of common stock for U.S. federal income tax purposes and a holder of Pre-Funded Warrants should generally be taxed in the same manner as a holder of common stock, as described below. Accordingly, no gain or loss should be recognized upon the exercise of a Pre-Funded Warrant and, upon exercise, the holding period of a Pre-Funded Warrant should carry over to the share of common stock received. Similarly, we believe that the tax basis of the Pre-Funded Warrant should carry over to the share of common stock received upon exercise, increased by the exercise price of $0.001 per share.
Each holder should consult his, her or its own tax advisor regarding the risks associated with the acquisition of Pre-Funded Warrants pursuant to this offering (including potential alternative characterizations). The balance of this discussion generally assumes that the characterization described above is respected for U.S. federal income tax purposes.
Allocation of Purchase Price
For U.S. federal income tax purposes, each share of common stock (or, in lieu of common stock, each Pre-Funded Warrant) and the accompanying Common Warrants issued pursuant to this offering will be treated as an “investment unit” consisting of one share of common stock or one Pre-Funded Warrant (which, as described above, we believe should generally be treated as a share of common stock for U.S. federal income tax purposes), as applicable, and the accompanying Common Warrants, each to acquire one share of common stock. The purchase price for each investment unit will be allocated between these components in proportion to their relative fair market values at the time the unit is purchased by the holder. This allocation of the purchase price for each unit will establish the holder’s initial tax basis for U.S. federal income tax purposes in the share of common stock (or, in lieu of common stock, Pre-Funded Warrant) and the Common Warrant included in each unit.
We will not be providing holders with such allocation, and it is possible that different holders will reach different determinations regarding such allocation. A holder's allocation of purchase price between each share of common stock (or Pre-Funded Warrant) and the accompanying Common Warrants is not binding on the IRS or the courts, and no assurance can be given that the IRS or the courts will agree with a holder's allocation.
Each holder should consult his, her or its own tax advisor regarding the allocation of the purchase price between the common stock (or, in lieu of common stock, Pre-Funded Warrants) and the Common Warrants.
Tax Considerations Applicable to U.S. Holders
Exercise and Expiration of Common Warrants
Except as discussed below with respect to the cashless exercise of a Common Warrant, a U.S. Holder generally will not recognize gain or loss for U.S. federal income tax purposes upon exercise of a Common Warrant. The U.S. Holder will take a tax basis in the shares acquired on the exercise of a Common Warrant equal to the exercise price of the Common Warrant, increased by the U.S. Holder’s adjusted tax basis in the Common Warrant exercised (as determined pursuant to the rules discussed above). The U.S. Holder’s holding period in the shares of common stock acquired on the exercise of a Common Warrant will begin on the date of exercise or possibly the day after such exercise, and will not include the period for which the U.S. Holder held the Common Warrant.
The lapse or expiration of an unexercised Common Warrant will be treated as if the U.S. Holder sold or exchanged the Common Warrant and recognized a capital loss equal to the U.S. Holder’s tax basis in the Common Warrant. The deductibility of capital losses is subject to limitations.
The tax consequences of a cashless exercise of a Common Warrant are not clear under current U.S. tax law. A cashless exercise may be tax-free, either because the exercise is not a realization event or because the exercise is treated as a recapitalization for U.S. federal income tax purposes. In either tax-free situation, a U.S. Holder’s tax basis in the common stock received generally would equal the U.S. Holder’s tax basis in the Common Warrants. If the cashless exercise was not a realization event, it is unclear whether a U.S. Holder’s holding period for the common stock would be treated as commencing on the date of exercise of the Common Warrant or the day following the date of exercise of the Common Warrant. If the cashless exercise were treated as a recapitalization, the holding period of the common stock would include the holding period of the Common Warrants.
It is also possible that a cashless exercise could be treated as a taxable exchange in which gain or loss would be recognized. In such event, a U.S. Holder could be deemed to have surrendered Common Warrants having an aggregate fair market value equal to the exercise price for the total number of Common Warrants to be exercised. The U.S. Holder would recognize capital gain or loss in an amount equal to the difference between the fair market value of the common stock received in respect of the Common Warrants deemed surrendered and the U.S. Holder’s tax basis in such common warrants. Such gain or loss would be long-term or short-term, depending on the U.S. Holder’s holding period in the Common Warrants deemed surrendered. In this case, a U.S. Holder’s tax basis in the common stock received would equal the sum of the U.S. Holder’s initial investment in the exercised Common Warrants (i.e., the portion of the U.S. Holder’s purchase price for the investment unit that is allocated to the Common Warrants, as described above under “Allocation of Purchase Price”) and the exercise price of such Common Warrants. It is unclear whether a U.S. Holder’s holding period for the common stock would commence on the date of exercise of the Common Warrant or the day following the date of exercise of the Common Warrant. There may also be alternative characterizations of any such taxable exchange that would result in similar tax consequences, except that a U.S. Holder’s gain or loss would be short-term.
Due to the absence of authority on the U.S. federal income tax treatment of a cashless exercise, there can be no assurance which, if any, of the alternative tax consequences and holding periods described above would be adopted by the IRS or a court of law. Accordingly, U.S. Holders should consult their tax advisors regarding the tax consequences of a cashless exercise of the Common Warrants.
Distributions
As discussed above, we currently anticipate that we will retain future earnings, if any, to finance the growth and development of our business and do not intend to pay cash dividends in respect of shares of common stock in the foreseeable future. In the event that we do make distributions on our common stock to a U.S. Holder, those distributions generally will constitute dividends for U.S. tax purposes to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). Distributions in excess of our current and accumulated earnings and profits will constitute a return of capital that is applied against and reduces, but not below zero, a U.S. Holder’s adjusted tax basis in our common stock. Any remaining excess will be treated as gain realized on the sale or exchange of shares of common stock as described below under the section titled “—Disposition of common stock, Pre-Funded Warrants or Common Warrants.”
Subject to applicable limitations, dividends paid to certain non-corporate U.S. Holders may be eligible for taxation as “qualified dividend income” and therefore may be taxable at rates applicable to long-term capital gains. U.S. Holders should consult their tax advisors regarding the availability of the reduced tax rate on dividends in their particular circumstances. Dividends received by a corporate U.S. Holder may be eligible for the dividends-received deduction if the U.S. Holder meets certain holding period and other applicable requirements.
Certain Adjustments to Pre-Funded Warrants or Common Warrants
The number of shares of common stock issued upon the exercise of the Pre-Funded Warrants or Common Warrants and the exercise price of Pre-Funded Warrants or Common Warrants are subject to adjustment in certain circumstances. Adjustments (or failure to make adjustments) that have the effect of increasing a U.S. Holder’s proportionate interest in our assets or earnings and profits may, in some circumstances, result in a constructive distribution to the U.S. Holder. Adjustments to the conversion rate made pursuant to a bona fide reasonable adjustment formula which has the effect of preventing the dilution of the interest of the holders of Pre-Funded Warrants or Common Warrants generally should not be deemed to result in a constructive distribution. If an adjustment is made that does not qualify as being made pursuant to a bona fide reasonable adjustment formula, a U.S. Holder of Pre-Funded Warrants or Common Warrants may be deemed to have received a constructive distribution from us, even though such U.S. Holder has not received any cash or property as a result of such adjustment. The tax consequences of the receipt of a distribution from us are described above under “Distributions.”
Disposition of Common Stock, Pre-Funded Warrants or Common Warrants
Upon a sale or other taxable disposition (other than a redemption treated as a distribution, which will be taxed as described above under “Distributions”) of shares of common stock, Pre-Funded Warrants or Common Warrants, a U.S. Holder generally will recognize capital gain or loss in an amount equal to the difference between the amount realized upon the disposition and the U.S. Holder’s adjusted tax basis in the common stock, Pre-Funded Warrants or Common Warrants sold. Capital gain or loss will constitute long-term capital gain or loss if the U.S. Holder’s holding period for the common stock, Pre-Funded Warrants or Common Warrants exceeds one year at the time of the disposition. The deductibility of capital losses is subject to certain limitations. U.S. Holders who recognize losses with respect to a disposition of shares of common stock, Pre-Funded Warrants or Common Warrants should consult their own tax advisors regarding the tax treatment of such losses.
Information Reporting and Backup Withholding
Information reporting requirements generally will apply to payments of distributions (including constructive distributions) on the common stock, Pre-Funded Warrants and Common Warrants and to the proceeds of a sale or other disposition of common stock, Pre-Funded Warrants and Common Warrants paid by us to a U.S. Holder unless such U.S. Holder is an exempt recipient, such as a corporation. Backup withholding will apply to those payments if the U.S. Holder fails to provide the holder’s taxpayer identification number, or certification of exempt status, or if the holder otherwise fails to comply with applicable requirements to establish an exemption.
Backup withholding is not an additional tax. Rather, any amounts withheld under the backup withholding rules will be allowed as a refund or a credit against the U.S. Holder’s U.S. federal income tax liability provided the required information is timely furnished to the IRS. U.S. Holders should consult their own tax advisors regarding their qualification for an exemption from information reporting and backup withholding and the procedure for obtaining such an exemption.
Tax Considerations Applicable to Non-U.S. Holders
Exercise and Expiration of Common Warrants
In general, a Non-U.S. Holder will not recognize gain or loss for U.S. federal income tax purposes upon the exercise of Common Warrants into shares of common stock, however, to the extent a cashless exercise results in a taxable exchange, the consequences would be similar to those described in the discussion below under “Disposition of Common Stock; Pre-Funded Warrants or Common Warrants”.
The expiration of a Common Warrant will be treated as if the Non-U.S. Holder sold or exchanged the Common Warrant and recognized a capital loss equal to the Non-U.S. Holder’s tax basis in the Common Warrant. However, a Non-U.S. Holder will not be able to utilize a loss recognized upon expiration of a Common Warrant against the Non-U.S. Holder’s U.S. federal income tax liability unless the loss is effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States (and, if an income tax treaty applies, is attributable to a permanent establishment or fixed base in the United States) or is treated as a U.S.-source loss and the Non-U.S. Holder is present 183 days or more in the taxable year of disposition and certain other conditions are met.
Certain Adjustments to Warrants
As described under “—U.S. Holders—Certain Adjustments to Pre-Funded Warrants or Common Warrants,” an adjustment to the Pre-Funded Warrants or Common Warrants could result in a constructive distribution to a Non-U.S. Holder, which would be treated as described under “Distributions” below. Any resulting withholding tax attributable to deemed dividends would be collected from other amounts payable or distributable to the Non-U.S. Holder. Non-U.S. Holders should consult their tax advisors regarding the proper treatment of any adjustments to the Pre-Funded Warrants or Common Warrants.
In addition, regulations governing “dividend equivalents” under Section 871(m) of the Code may apply to the Pre-Funded Warrants. Under those regulations, an implicit or explicit payment under the Pre-Funded Warrants that references a dividend distribution on our common stock would possibly be taxable to a Non-U.S. Holder as described under “Distributions” below. Such dividend equivalent amount would be taxable and subject to withholding whether or not there is actual payment of cash or other property, and the Company may satisfy any withholding obligations it has in respect of the Pre-Funded Warrants by withholding from other amounts due to the Non-U.S. Holder. Non-U.S. Holders are encouraged to consult their own tax advisors regarding the application of Section 871(m) of the Code to the Pre-Funded Warrants.
Distributions
As discussed above, we currently anticipate that we will retain future earnings, if any, to finance the growth and development of our business and do not intend to pay cash dividends in respect of our common stock in the foreseeable future. In the event that we do make distributions on our common stock to a Non-U.S. Holder, those distributions generally will constitute dividends for U.S. federal income tax purposes as described in “—U.S. Holders—Distributions.” To the extent those distributions do not constitute dividends for U.S. federal income tax purposes (i.e., the amount of such distributions exceeds both our current and our accumulated earnings and profits), they will constitute a return of capital and will first reduce a Non-U.S. Holder’s basis in our common stock (determined separately with respect to each share of common stock), but not below zero, and then will be treated as gain from the sale of that common stock as described below under the section titled “—Disposition of Common Stock, Pre-Funded Warrants or Common Warrants.”
Any distribution (including constructive distributions) on shares of common stock that is treated as a dividend paid to a Non-U.S. Holder that is not effectively connected with the holder’s conduct of a trade or business in the United States will generally be subject to withholding tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and the Non-U.S. Holder’s country of residence. To obtain a reduced rate of withholding under a treaty, a Non-U.S. Holder generally will be required to provide the applicable withholding agent with a properly executed IRS Form W-8BEN, IRS Form W-8BEN-E or other appropriate form, certifying the Non-U.S. Holder’s entitlement to benefits under that treaty. Such form must be provided prior to the payment of dividends and must be updated periodically. If a Non-U.S. Holder holds stock through a financial institution or other agent acting on the holder’s behalf, the holder will be required to provide appropriate documentation to such agent. The holder’s agent may then be required to provide certification to the applicable withholding agent, either directly or through other intermediaries. If you are eligible for a reduced rate of U.S. withholding tax under an income tax treaty, you should consult with your own tax advisor to determine if you are able to obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim for a refund with the IRS.
We generally are not required to withhold tax on dividends paid (or constructive dividends deemed paid) to a Non-U.S. Holder that are effectively connected with the holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment or fixed base that the holder maintains in the United States) if a properly executed IRS Form W-8ECI, stating that the dividends are so connected, is furnished to us (or, if stock is held through a financial institution or other agent, to the applicable withholding agent). In general, such effectively connected dividends will be subject to U.S. federal income tax on a net income basis at the regular tax rates applicable to U.S. persons. A corporate Non-U.S. Holder receiving effectively connected dividends may also be subject to an additional “branch profits tax,” which is imposed, under certain circumstances, at a rate of 30% (or such lower rate as may be specified by an applicable treaty) on the corporate Non-U.S. Holder’s effectively connected earnings and profits, subject to certain adjustments.
See also the sections below titled “—Backup Withholding and Information Reporting” and “—Foreign Accounts” for additional withholding rules that may apply to dividends paid to certain foreign financial institutions or non-financial foreign entities.
Disposition of Common Stock, Pre-Funded Warrants or Common Warrants
Subject to the discussions below under the sections titled “—Backup Withholding and Information Reporting” and “—Foreign Accounts,” a Non-U.S. Holder generally will not be subject to U.S. federal income or withholding tax with respect to gain recognized on a sale or other disposition (other than a redemption treated as a distribution, which will be taxable as described above under “Distributions”) of shares of common stock, Pre-Funded Warrants or Common Warrants unless:
● the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business in the United States, and if an applicable income tax treaty so provides, the gain is attributable to a permanent establishment or fixed base maintained by the Non-U.S. Holder in the United States; in these cases, the Non-U.S. Holder will be taxed on a net income basis at the regular tax rates and in the manner applicable to U.S. persons, and, if the Non-U.S. Holder is a corporation, an additional branch profits tax at a rate of 30%, or a lower rate as may be specified by an applicable income tax treaty, may also apply;
● the Non-U.S. Holder is a nonresident alien present in the United States for 183 days or more in the taxable year of the disposition and certain other requirements are met, in which case the Non-U.S. Holder will be subject to a 30% tax (or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder’s country of residence) on the net gain derived from the disposition, which may be offset by certain U.S.-source capital losses of the Non-U.S. Holder, if any; or
● the common stock constitutes a U.S. real property interest because we are, or have been at any time during the five-year period preceding such disposition (or the Non-U.S. Holder’s holding period of the common stock, Pre-Funded Warrants or Common Warrants, if shorter), a “U.S. real property holding corporation,” unless the common stock is regularly traded on an established securities market, as defined by applicable Treasury Regulations, and the Non-U.S. Holder held no more than 5% of our outstanding common stock, directly or indirectly, during the shorter of the five-year period ending on the date of the disposition or the period that the Non-U.S. Holder held the common stock. Special rules may apply to the determination of the 5% threshold in the case of a holder of Pre-Funded Warrants or Common Warrants. Non-U.S. Holders are urged to consult their own tax advisors regarding the effect of holding Pre-Funded Warrants or Common Warrants on the calculation of such 5% threshold. Generally, a corporation is a “U.S. real property holding corporation” if the fair market value of its “U.S. real property interests” (as defined in the Code and applicable regulations) equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. Although there can be no assurance, we believe that we are not currently, and we do not anticipate becoming, a “U.S. real property holding corporation” for U.S. federal income tax purposes. No assurance can be provided that the common stock will be regularly traded on an established securities market for purposes of the rules described above. Non-U.S. Holders are urged to consult their own tax advisors regarding the U.S. federal income tax considerations that could result if we are, or become, a “U.S. real property holding corporation.
See the sections titled “—Backup Withholding and Information Reporting” and “—Foreign Accounts” for additional information regarding withholding rules that may apply to proceeds of a disposition of the common stock, Pre-Funded Warrants or Common Warrants paid to foreign financial institutions or non-financial foreign entities.
Backup Withholding and Information Reporting
We must report annually to the IRS and to each Non-U.S. Holder the gross amount of the distributions (including constructive distributions) on the common stock, Pre-Funded Warrants or Common Warrants paid to such holder and the tax withheld, if any, with respect to such distributions. Non-U.S. Holders may have to comply with specific certification procedures to establish that the holder is not a U.S. person (as defined in the Code) in order to avoid backup withholding at the applicable rate, currently 24%, with respect to dividends (or constructive dividends) on the common stock, Pre-Funded Warrants or Common Warrants. Generally, a holder will comply with such procedures if it provides a properly executed IRS Form W-8BEN (or other applicable Form W-8) or otherwise meets documentary evidence requirements for establishing that it is a Non-U.S. Holder, or otherwise establishes an exemption. Dividends paid to Non-U.S. Holders subject to withholding of U.S. federal income tax, as described above under the heading “Distributions,” will generally be exempt from U.S. backup withholding.
Information reporting and backup withholding generally will apply to the proceeds of a disposition of the common stock, Pre-Funded Warrants or Common Warrants by a Non-U.S. Holder effected by or through the U.S. office of any broker, U.S. or foreign, unless the holder certifies its status as a Non-U.S. Holder and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding will not apply to a payment of disposition proceeds to a Non-U.S. Holder where the transaction is effected outside the United States through a non-U.S. office of a broker. However, for information reporting purposes, dispositions effected through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through a U.S. office of a broker. Non-U.S. Holders should consult their own tax advisors regarding the application of the information reporting and backup withholding rules to them.
Copies of information returns may be made available to the tax authorities of the country in which the Non-U.S. Holder resides or is incorporated under the provisions of a specific treaty or agreement.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a Non-U.S. Holder can be refunded or credited against the Non-U.S. Holder’s U.S. federal income tax liability, if any, provided that an appropriate claim is timely filed with the IRS.
Foreign Accounts
The Foreign Account Tax Compliance Act, or FATCA, generally imposes a 30% withholding tax on dividends (including constructive dividends) on the common stock, Pre-Funded Warrants and Common Warrants if paid to a non-U.S. entity unless (i) if the non-U.S. entity is a “foreign financial institution,” the non-U.S. entity undertakes certain due diligence, reporting, withholding, and certification obligations, (ii) if the non-U.S. entity is not a “foreign financial institution,” the non-U.S. entity identifies certain of its U.S. investors, if any, or (iii) the non-U.S. entity is otherwise exempt under FATCA.
Withholding under FATCA generally will apply to payments of dividends (including constructive dividends) on our common stock, Pre-Funded Warrants and Common Warrants. While withholding under FATCA would have also applied to payments of gross proceeds from a sale or other disposition of the common stock, Pre-Funded Warrants or Common Warrants, under proposed U.S. Treasury Regulations withholding on payments of gross proceeds is not required. Although such regulations are not final, applicable withholding agents may rely on the proposed regulations until final regulations are issued.
An intergovernmental agreement between the United States and an applicable foreign country may modify the requirements described in this section. Under certain circumstances, a holder may be eligible for refunds or credits of the tax. Holders should consult their own tax advisors regarding the possible implications of FATCA on their investment in the common stock, Pre-Funded Warrants or Common Warrants.
Federal Estate Tax
Common stock owned or treated as owned by an individual who is not a citizen or resident of the United States (as specially defined for U.S. federal estate tax purposes) at the time of death will be included in the individual’s gross estate for U.S. federal estate tax purposes and, therefore, may be subject to U.S. federal estate tax, unless an applicable estate tax or other treaty provides otherwise. The foregoing may also apply to Common Warrants and Pre-Funded Warrants. A Non-U.S. Holder should consult his, her, or its own tax advisor regarding the U.S. federal estate tax consequences of the ownership or disposition of shares of the common stock, Pre-Funded Warrants and Common Warrants.
The preceding discussion of material U.S. federal tax considerations is for informational purposes only. It is not tax advice. Prospective investors should consult their own tax advisors regarding the particular U.S. federal, state, local and non-U.S. tax consequences of purchasing, holding and disposing of the common stock, Pre-Funded Warrants or Common Warrants, including the consequences of any proposed changes in applicable laws.
UNDERWRITING
We are offering the securities described in this prospectus through the underwriters named below. Ladenburg Thalmann & Co. Inc. is acting as the representative (the “Representative”) of the underwriters in this offering. Subject to the terms and conditions of the underwriting agreement, the underwriters have agreed to purchase the number of our securities set forth opposite its name below.
|
Underwriters |
Number of Common Stock Units |
Number of PFW Units
|
||||||
|
Ladenburg Thalmann & Co. Inc. |
||||||||
|
Total |
||||||||
A copy of the underwriting agreement has been filed as an exhibit to the registration statement of which this prospectus is part.
We have been advised by the underwriters that they propose to offer the Common Stock Units and PFW Units directly to the public at the public offering prices set forth on the cover page of this prospectus. Any securities sold by the underwriters to securities dealers will be sold at the public offering price less a selling concession not in excess of $____ per Common Stock Unit and $____ per PFW Unit.
The underwriting agreement provides that the underwriters’ obligation to purchase the securities we are offering is subject to conditions contained in the underwriting agreement.
No action has been taken by us or the underwriters that would permit a public offering of the Common Stock Units and PFW Units in any jurisdiction outside the United States where action for that purpose is required. None of our securities included in this offering may be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sales of any of the securities offering hereby be distributed or published in any jurisdiction except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons who receive this prospectus are advised to inform themselves about and to observe any restrictions relating to this offering of securities and the distribution of this prospectus. This prospectus is neither an offer to sell nor a solicitation of any offer to buy the securities in any jurisdiction where that would not be permitted or legal.
The underwriters have advised us that they do not intend to confirm sales to any account over which they exercise discretionary authority.
Underwriting Discount and Expenses
The following table summarizes the underwriting discount and commission to be paid to the underwriters by us.
|
Per Common Stock Unit(1) |
Per |
Total |
Total |
|||||||||||||
|
Public offering price |
$ | $ | $ | $ | ||||||||||||
|
Underwriting discounts and commissions to be paid to underwriters by us(3)(4) |
$ | $ | $ | $ | ||||||||||||
|
Proceeds, before expenses, to us |
$ | $ | $ | $ | ||||||||||||
________________
|
(1) |
The public offering price and underwriting discount in respect of the Common Stock Units corresponds to a public offering price per share of common stock and accompanying Common Warrant of $____ ($____ net of the underwriting discount). |
|
(2) |
The public offering price and underwriting discount in respect of the PFW Units corresponds to a public offering price per PFW Unit of $____ ($____ net of the underwriting discount). |
|
(3) |
We have also agreed to reimburse the accountable expenses of the representative, including a pre-closing expense allowance of up to a maximum of $30,000 and an additional closing expense allowance up to a maximum of $85,000. |
|
(4) |
We have granted the underwriters an option for a period of up to 45 days from the date of this prospectus to purchase up to 104,822 additional shares of our common stock and/or Series A warrants to purchase up to an additional 104,822 shares of our common stock, or any combination thereof, as determined by the underwriters, at the assumed public offering price, less underwriting discounts and commissions, in each case solely to cover over-allotments, if any. |
We estimate the total expenses payable by us for this offering to be approximately $1,020,000, which amount includes (i) the underwriting discount of $800,000, (ii) reimbursement of the accountable expenses of the underwriters, including the legal fees of the representative, in an amount not to exceed a total of $115,000 consisting of: $30,000 for pre-closing expenses plus $85,000 for closing expenses and (iii) other estimated company expenses of approximately $105,000, which includes legal, accounting, printing costs, and various fees associated with the registration and listing of our shares.
The securities we are offering are being offered by the underwriters subject to certain conditions specified in the underwriting agreement.
Over-allotment Option
We have granted the underwriters an option for a period of up to 45 days from the date of this prospectus to purchase up to 104,822 additional shares of our common stock and/or Series A warrants to purchase up to an additional 104,822 shares of our common stock, or any combination thereof, as determined by the underwriters, at the assumed public offering price, less underwriting discounts and commissions.
The underwriters may exercise the option solely to cover overallotments, if any, made in connection with this offering. If any additional shares of common stock and/or Series A warrants are purchased, the underwriters will offer these shares of common stock and/or Series A warrants on the same terms as those on which the other securities are being offered.
Representative Warrants
Upon completion of this offering, we have agreed to issue to the representative as compensation, warrants to purchase up to 41,928 shares of common stock (or 48,218 shares of common stock assuming the exercise of the over-allotment option in full), which equals 6.0% of the aggregate number of the Shares and the shares of common stock underlying the Pre-Funded Warrants sold in this offering, but excluding shares of Common Stock underlying the Series A Warrants (the “Representative Warrants”). The Representative Warrants will be exercisable at a per share exercise price equal to 155% of the public offering price per Common Stock Unit in this offering. The Representative Warrants are exercisable at any time and from time to time, in whole or in part, during the four and one half year period commencing 180 days following the commencement of sales of the securities issued in this offering.
The Representative Warrants have been deemed compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to Rule 5110(e)(1)(A) of FINRA. The representative of the Underwriters (or permitted assignees under Rule 5110(e)(2)) will not sell, transfer, assign, pledge, or hypothecate these Representative Warrants or the shares of common stock underlying these warrants, nor will they engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the warrants or the underlying securities for a period of 180 days following the commencement of sales of the securities issued in this offering.. The exercise price and number of shares issuable upon exercise of the Representative Warrants may be adjusted in certain circumstances including in the event of a stock dividend or our recapitalization, reorganization, merger or consolidation. However, the exercise price of the Representative Warrants or the underlying shares of common stock will not be adjusted for issuances of shares of common stock at a price below the warrant exercise price.
Right of First Refusal
We have granted to Ladenburg Thalmann & Co. Inc. (“Ladenburg”) the right of first refusal for a period of nine (9) months following the closing of this offering the opportunity to participate as a sole bookrunner or exclusive placement agent or exclusive sales agent in respect of any future financing by us on terms and conditions mutually acceptable to us and Ladenburg.
Fee Obligation
Within nine (9) months after the date of termination or expiration of the engagement agreement that we entered into with Ladenburg dated May 21, 2024, the securities or securities convertible into or exchangeable for the securities that are sold by us to investors contacted by Ladenburg, then we shall pay to Ladenburg, at the time of each such sale or transaction, the fees set forth above with respect to any such sale.
Listing
Our shares of common stock are listed on the Nasdaq Capital Market under the symbol “AMIX.”
The last reported sale price of our common stock on October 31, 2024 was $14.31 per share. The actual public offering price per Common Stock Unit or PFW Unit, as the case may be, will be determined between us, the underwriters and the investors in the offering, and may be at a discount to the current market price of our common stock. Therefore, the assumed public offering price used throughout this prospectus may not be indicative of the final offering price. There is no established public trading market for the Common Warrants or Pre-Funded Warrants, and we do not expect such a market to develop. In addition, we do not intend to apply for a listing of the Common Warrants, Pre-Funded Warrants or the Representative Warrants on any national securities exchange or other nationally recognized trading system.
See “Prospectus Summary” for information about the listing of our common stock.
Lock-up Agreements
Our officers, directors and each of their respective affiliates and associated partners have agreed with the underwriters to be subject to a lock-up period of 90 days following the date of this prospectus. This means that, during the applicable lock-up period, such persons may not offer for sale, contract to sell, sell, distribute, grant any option, right or warrant to purchase, pledge, hypothecate or otherwise dispose of, directly or indirectly, any shares of our common stock or any securities convertible into, or exercisable or exchangeable for, shares of our common stock. Certain limited transfers are permitted during the lock-up period if the transferee agrees to these lock-up restrictions. We have also agreed, in the underwriting agreement, to similar lock-up restrictions on the issuance and sale of our securities for 90 days following the closing of this offering, although we will be permitted to issue stock options or stock awards to directors, officers and employees under our existing plans. The Representative may, in its sole discretion and without notice, waive the terms of any of these lock-up agreements.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Equity Stock Transfer, LLC.
Stabilization, Short Positions and Penalty Bids
The underwriters may engage in syndicate covering transactions stabilizing transactions and penalty bids or purchases for the purpose of pegging, fixing or maintaining the price of our common stock:
|
• |
Syndicate covering transactions involve purchases of securities in the open market after the distribution has been completed in order to cover syndicate short positions. Such a naked short position would be closed out by buying securities in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the securities in the open market after pricing that could adversely affect investors who purchase in the offering. |
|
• |
Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specific maximum. |
|
• |
Penalty bids permit the underwriters to reclaim a selling concession from a syndicate member when the securities originally sold by the syndicate member are purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
These syndicate covering transactions, stabilizing transactions, and penalty bids may have the effect of raising or maintaining the market prices of our securities or preventing or retarding a decline in the market prices of our securities. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the price of our common stock. These transactions may be effected on the Nasdaq Stock Market, in the over-the-counter market or on any other trading market and, if commenced, may be discontinued at any time.
In connection with this offering, the underwriters also may engage in passive market making transactions in our common stock in accordance with Regulation M during a period before the commencement of offers or sales of shares of our common stock in this offering and extending through the completion of the distribution. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for that security. However, if all independent bids are lowered below the passive market maker’s bid that bid must then be lowered when specific purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Neither we, nor the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the prices of our securities. In addition, neither we nor the underwriters make any representation that the underwriters will engage in these transactions or that any transactions, once commenced will not be discontinued without notice.
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including certain liabilities arising under the Securities Act, or to contribute to payments that the underwriters may be required to make for these liabilities.
Other
From time to time, certain of the underwriters and/or their affiliates may in the future provide, various investment banking and other financial services for us for which they may receive customary fees. In the course of their businesses, the underwriters and their affiliates may actively trade our securities or loans for their own account or for the accounts of customers, and, accordingly, the underwriters and their affiliates may at any time hold long or short positions in such securities or loans. Except for services provided in connection with this offering, no underwriter has provided any investment banking or other financial services to us during the 180-day period preceding the date of this prospectus and we do not expect to retain any underwriter to perform any investment banking or other financial services for at least 90 days after the date of this prospectus.
Electronic Distribution
A prospectus in electronic format may be made available on the websites maintained by the underwriters, if any, participating in this offering and the underwriters may distribute prospectuses electronically. Other than the prospectus in electronic format, the information on these websites is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us or the underwriters, and should not be relied upon by investors.
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer to the offeree under this prospectus.
LEGAL MATTERS
The validity of the securities offered hereby will be passed upon for us by ArentFox Schiff LLP, Washington, DC. The underwriters are being represented by Blank Rome LLP, New York, New York in connection with this offering.
EXPERTS
The financial statements of Autonomix Medical, Inc. as of March 31, 2024 and 2023 and for each of the years in the two-year period ended March 31, 2024, have been audited by Forvis Mazars, LLP, independent registered public accounting firm, as set forth in their report thereon, included in this registration statement. Such financial statements have been included herein in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The report of Forvis Mazars, LLP contains an explanatory paragraph regarding substantial doubt about the Company’s ability to continue as a going concern.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act for the securities being offered by this prospectus. This prospectus, which is part of the registration statement, does not contain all of the information included in the registration statement and the exhibits. For further information about us and the securities offered by this prospectus, you should refer to the registration statement and its exhibits. References in this prospectus to any of our contracts or other documents are not necessarily complete, and you should refer to the exhibits attached to the registration statement for copies of the actual contract or document. SEC filings are also available to the public at the SEC’s website at www.sec.gov.
We are subject to the reporting and information requirements of the Exchange Act and, as a result, we file periodic and current reports, proxy statements and other information with the SEC. We make our periodic reports and other information filed with or furnished to the SEC, available, free of charge, through our website as soon as reasonably practicable after those reports and other information are filed with or furnished to the SEC. Additionally, these periodic reports, proxy statements and other information are available at the SEC’s website at www.sec.gov.
REVERSE SPLIT FINANCIAL DATA:
On October 23, 2024, the Company announced that it is filing a certificate of amendment to its articles of incorporation with the Secretary of State of the State of Delaware to effect a 1-for-20 reverse stock split of its common stock. The table below sets forth the impact of the Reverse Stock Split on the Company's net loss per common share - basic and diluted; weighted average common shares outstanding - basic and diluted; and shares issued and outstanding, for the years ended March 31, 2024 and 2023; and the three months ended June 30, 2024 and 2023:
|
Pre Split (1) |
Post Split |
|||||||||||||||
|
Year Ended March 31, |
Year Ended March 31, |
|||||||||||||||
|
2024 |
2023 |
2024 |
2023 |
|||||||||||||
|
Net loss (in thousands, except share and per share data) |
$ | (15,426 | ) | $ | (1,990 | ) | $ | (15,426 | ) | $ | (1,990 | ) | ||||
|
Net loss per common share - basic |
$ | (1.05 | ) | $ | (0.17 | ) | $ | (21.09 | ) | $ | (3.31 | ) | ||||
|
Net loss per common share - diluted |
$ | (1.05 | ) | $ | (0.17 | ) | $ | (21.09 | ) | $ | (3.31 | ) | ||||
|
Weighted average common shares outstanding - basic |
14,626,282 | 12,023,112 | 731,372 | 601,166 | ||||||||||||
|
Weighted average common shares outstanding - diluted |
14,626,282 | 12,023,112 | 731,372 | 601,166 | ||||||||||||
|
Pre Split (1) |
Post Split |
|||||||||||||||
|
3 Months Ended June 30, |
3 Months Ended June 30, |
|||||||||||||||
|
2024 |
2023 |
2024 |
2023 |
|||||||||||||
|
Net loss (in thousands, except share and per share data) |
$ | (2,699 | ) | $ | (865 | ) | $ | (2,699 | ) | $ | (865 | ) | ||||
|
Net loss per common share - basic |
$ | (0.14 | ) | $ | (0.07 | ) | $ | (2.85 | ) | $ | (1.34 | ) | ||||
|
Net loss per common share - diluted |
$ | (0.14 | ) | $ | (0.07 | ) | $ | (2.85 | ) | $ | (1.34 | ) | ||||
|
Weighted average common shares outstanding - basic |
18,902,248 | 12,884,604 | 945,382 | 644,241 | ||||||||||||
|
Weighted average common shares outstanding - diluted |
18,902,248 | 12,884,604 | 945,382 | 644,241 | ||||||||||||
(1) The pre-split amounts represent the amounts reported in the Company's Annual Report on Form 10-K or Quarterly Report on Form 10-Q for the respective periods.
Index to Financial Statements
|
Page |
|
|
Report of Independent Registered Public Accounting Firm |
89 |
|
Balance Sheets as of March 31, 2024 and 2023 |
90 |
|
Statements of Operations for the years ended March 31, 2024 and 2023 |
91 |
|
Statements of Changes in Stockholders' Equity for the years ended March 31, 2024 and 2023 |
92 |
|
Statements of Cash Flows for the years ended March 31, 2024 and 2023 |
93 |
|
Notes to Financial Statements |
94 |
|
Unaudited Condensed Balance Sheets as of June 30, 2024 and March 31, 2024 |
111 |
|
Unaudited Condensed Statements of Operations for the three months ended June 30, 2024 and 2023 |
112 |
|
Unaudited Condensed Statements of Changes in Stockholders' Equity for the three months ended June 30, 2024 and 2023 |
113 |
|
Unaudited Condensed Statements of Cash Flows for the three months ended June 30, 2024 and 2023 |
114 |
|
Notes to the Unaudited Condensed Financial Statements |
115 |
Report of Independent Registered Public Accounting Firm
To the Shareholders, Board of Directors, and Audit Committee
Autonomix Medical, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Autonomix Medical, Inc. (the “Company”) as of March 31, 2024 and 2023, the related statements of operations, changes in stockholders’ equity, and cash flows for each of the years in the two-year period ended March 31, 2024, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of March 31, 2024 and 2023, and the results of its operations and its cash flows for each of the years in the two-year period ended March 31, 2024, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has an accumulated deficit since inception, has not generated revenue from operations, and does not expect to experience positive cash flows from operating activities in the near term. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to this matter are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits.
We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures include examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements.
Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ FORVIS, LLP
We have served as the Company’s auditor since 2022.
Atlanta, Georgia
May 31, 2024
Balance Sheets
|
(in thousands, except par value and share data) |
As of |
|||||||
|
March 31, |
||||||||
|
2024 |
2023 |
|||||||
|
Assets |
||||||||
|
Current assets: |
||||||||
|
Cash |
$ | 8,608 | $ | 865 | ||||
|
Other current assets |
783 | — | ||||||
|
Total current assets |
9,391 | 865 | ||||||
|
Long-term assets: |
||||||||
|
Fixed assets, net |
16 | — | ||||||
|
Total long-term assets |
16 | — | ||||||
|
Total Assets |
$ | 9,407 | $ | 865 | ||||
|
Liabilities and Stockholders' Equity |
||||||||
|
Current liabilities: |
||||||||
|
Accounts payable |
$ | 492 | $ | 173 | ||||
|
Accrued expenses |
285 | 48 | ||||||
|
Total current liabilities |
777 | 221 | ||||||
|
Long-term liabilities: |
||||||||
|
Long term debt - convertible notes, net of unamortized debt discount |
1,002 | — | ||||||
|
Total long-term liabilities |
1,002 | — | ||||||
|
Total Liabilities |
$ | 1,779 | $ | 221 | ||||
|
Commitments and contingencies (Note 5) |
||||||||
|
Stockholders' equity: |
||||||||
|
Preferred stock, $0.001 par value, 10,000,000 shares authorized as of March 31, 2024, no shares issued and outstanding, and 7,100,000 shares authorized as of March 31, 2023, no shares issued and outstanding |
$ | — | $ | — | ||||
|
Common stock, $0.001 par value, 500,000,000 shares authorized as of March 31, 2024, 18,846,094 shares issued and outstanding, and 25,000,000 shares authorized as of March 31, 2023, 12,336,571 shares issued and outstanding |
19 | 12 | ||||||
|
Additional paid-in capital |
46,578 | 24,175 | ||||||
|
Accumulated deficit |
(38,969 | ) | (23,543 | ) | ||||
|
Total Stockholders' Equity |
7,628 | 644 | ||||||
|
Total Liabilities and Stockholders' Equity |
$ | 9,407 | $ | 865 | ||||
See accompanying notes to the financial statements
Statements of Operations
|
For the Years Ended |
||||||||
|
March 31, |
||||||||
|
(in thousands, except share and per share data) |
2024 |
2023 |
||||||
|
Operating expenses: |
||||||||
|
General and administrative |
$ | 5,249 | $ | 1,245 | ||||
|
Research and development |
2,225 | 745 | ||||||
|
Warrant expense - termination agreement |
4,556 | — | ||||||
|
Total operating expenses |
12,030 | 1,990 | ||||||
|
Loss from operations |
(12,030 | ) | (1,990 | ) | ||||
|
Other (expense) income: |
||||||||
|
Warrant liability - mark-to-market |
(3,444 | ) | — | |||||
|
Interest expense |
(79 | ) | — | |||||
|
Interest income |
127 | — | ||||||
|
Total other expense |
(3,396 | ) | — | |||||
|
Loss before income taxes |
(15,426 | ) | (1,990 | ) | ||||
|
Income taxes |
— | — | ||||||
|
Net loss |
$ | (15,426 | ) | $ | (1,990 | ) | ||
|
Loss per share - basic and diluted |
$ | (1.05 | ) | $ | (0.17 | ) | ||
|
Weighted average shares outstanding - basic and diluted |
14,626,282 | 12,023,112 | ||||||
See accompanying notes to the financial statements
Statements of Changes in Stockholders' Equity
|
Additional |
Total |
|||||||||||||||||||||||||||
|
Preferred Stock |
Common Stock |
Paid-in |
Accumulated |
Stockholders' |
||||||||||||||||||||||||
|
(in thousands) |
Shares |
Amount |
Shares |
Amount |
Capital |
Deficit |
Equity |
|||||||||||||||||||||
|
Balance March 31, 2022 |
— | $ | — | 11,999 | $ | 12 | $ | 23,500 | $ | (21,553 | ) | $ | 1,959 | |||||||||||||||
|
Net loss |
— | — | — | — | — | (1,990 | ) | (1,990 | ) | |||||||||||||||||||
|
Issuance of common stock |
— | — | 338 | — | 675 | — | 675 | |||||||||||||||||||||
|
Balance March 31, 2023 |
— | — | 12,337 | 12 | 24,175 | (23,543 | ) | 644 | ||||||||||||||||||||
|
Net loss |
— | — | — | — | — | (15,426 | ) | (15,426 | ) | |||||||||||||||||||
|
Stock-based compensation |
— | — | — | — | 618 | — | 618 | |||||||||||||||||||||
|
Issuance of common stock |
— | — | 1,420 | 2 | 2,838 | — | 2,840 | |||||||||||||||||||||
|
Issuance of common stock from IPO, net of costs |
— | — | 2,234 | 2 | 9,873 | — | 9,875 | |||||||||||||||||||||
|
Issuance of common stock for extinguishment of convertible debt |
— | — | 335 | 1 | 499 | — | 500 | |||||||||||||||||||||
|
Issuance of common stock - warrants exercised |
— | — | 2,485 | 2 | (2 | ) | — | — | ||||||||||||||||||||
|
Issuance of restricted common stock |
— | — | 35 | — | — | — | — | |||||||||||||||||||||
|
Warrants issued for debt issuance costs |
— | — | — | — | 577 | — | 577 | |||||||||||||||||||||
|
Fair value of warrants issued - termination agreement |
— | — | — | — | 8,000 | — | 8,000 | |||||||||||||||||||||
|
Balance March 31, 2024 |
— | $ | — | 18,846 | $ | 19 | $ | 46,578 | $ | (38,969 | ) | $ | 7,628 | |||||||||||||||
See accompanying notes to the financial statements
Statements of Cash Flows
|
For the Years Ended March 31, |
||||||||
|
(in thousands) |
2024 |
2023 |
||||||
|
Cash Flows from Operating Activities: |
||||||||
|
Net loss |
$ | (15,426 | ) | $ | (1,990 | ) | ||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
|
Stock-based compensation |
618 | — | ||||||
|
Depreciation and amortization expense |
81 | — | ||||||
|
Warrant expense - termination agreement |
4,556 | — | ||||||
|
Warrant liability - mark-to-market |
3,444 | — | ||||||
|
Changes in operating assets - (increase)/decrease: |
||||||||
|
Other current assets |
(478 | ) | 9 | |||||
|
Changes in operating liabilities - increase: |
||||||||
|
Accounts payable |
320 | 81 | ||||||
|
Accrued expenses |
237 | 46 | ||||||
|
Net cash used in operating activities |
(6,648 | ) | (1,854 | ) | ||||
|
Cash Flows from Investing Activities: |
||||||||
|
Purchase of property and equipment |
(19 | ) | — | |||||
|
Net cash used in investing activities |
(19 | ) | — | |||||
|
Cash Flows from Financing Activities (increase/decrease): |
||||||||
|
Issuance of common stock |
2,840 | 675 | ||||||
|
Issuance of convertible debt |
2,000 | — | ||||||
|
Issuance of common stock from IPO |
10,866 | — | ||||||
|
IPO issuance costs |
(1,296 | ) | — | |||||
|
Net cash provided by financing activities |
14,410 | 675 | ||||||
|
Net change in cash and cash equivalents |
7,743 | (1,179 | ) | |||||
|
Cash and cash equivalents, at beginning of period |
865 | 2,044 | ||||||
|
Cash and cash equivalents, at end of period |
$ | 8,608 | $ | 865 | ||||
|
Supplemental cash flow disclosures: |
||||||||
|
Non-cash financing activities: |
||||||||
|
Warrants issued for debt issuance costs |
$ | 577 | $ | — | ||||
|
Proceeds from cashless exercise of warrants |
$ | 2 | $ | — | ||||
|
Fair value of warrants issued for issuance costs as part of IPO |
$ | 225 | $ | — | ||||
|
Holdback of IPO proceeds |
$ | 305 | $ | — | ||||
|
Convertible notes converted into common stock |
$ | 670 | $ | — | ||||
|
Settlement/conversion to common shares for debt issuance costs |
$ | (170 | ) | $ | — | |||
See accompanying notes to the financial statements
Notes to the Financial Statements
Note 1 – Description of the Business, Basis of Presentation and Summary of Significant Accounting Policies
Description of the Business
Autonomix Medical, Inc (“we,” “our,” the “Company”) is a medical device company organized as a Delaware corporation on June 10, 2014. The Company is a pre-revenue, clinical stage life sciences company focused on advancing innovative technologies for sensing and treating disorders relating to the peripheral nervous system.
Liquidity and Going Concern
The Company's financial statements are prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The Company is an early-stage company that is subject to all the risks associated with early-stage and emerging growth companies and has incurred losses since inception. For the years ended March 31, 2024 and 2023, the Company has net losses of approximately $15.4 million and $2.0 million, respectively and had net cash flows used in operating activities of $6.6 million and $1.9 million, respectively. The Company had no revenues for the years ended March 31, 2024 and 2023, respectively, accumulated deficit of $39.0 million and working capital of approximately $8.6 million as of March 31, 2024. The Company does not expect to generate positive cash flows from operating activities in the near future. These conditions, and the Company's ability to comply with such conditions, raise substantial doubt about the Company's ability to continue as a going concern within one year after the date that the financial statements are issued. The accompanying financial statements have been prepared on a going concern basis and do not include any adjustments that might be necessary if the Company is unable to continue as a going concern.
On January 26, 2024, the Company completed its initial public offering (“IPO”) of common stock. During the IPO, the Company sold a total of 2,234,222 shares of common stock at a purchase price of $5.00 per share for gross proceeds of $11.2 million and net proceeds of $9.8 million. On May 13, 2024, the Company cancelled 1,050 shares represented in the IPO due to payment disputes. As part of the IPO closing, $0.3 million was retained by the Company’s marketing partner as a holdback to be paid 90 days after the IPO. This $0.3 million was recorded in other current assets on the Company's balance sheet. In connection with the closing of the IPO, a portion of the Company’s convertible notes were converted into 335,000 shares of the Company’s common stock. Upon the closing of the IPO, certain notes were to be automatically converted according to their terms into the Company’s common stock to the extent and provided that certain holders of these notes are not permitted to convert such notes to the extent that the holders or any of its affiliates would beneficially own in excess of 4.99% of the Company’s common stock after such conversion. Due to this 4.99% limitation, principal representing $1.3 million, or 665,000 shares, of these convertible notes remained outstanding. The Company's existing cash resources, unexercised warrants and the cash received from the IPO are not expected to provide sufficient funds to carry out the Company's operations and clinical trials through the next twelve months.
The Company paid a cash commission of 7.0% to the selling agent on sales of the shares of common stock in the IPO. In addition, the Company has issued the selling agent warrants to purchase up to a total number of shares of common stock equal to 2.675% of the total number of shares sold in the IPO at an exercise price equal to 125% of the public offering price of the shares sold in the IPO. The selling agent warrants will be exercisable at any time, and from time to time, in whole or in part, commencing from the date that is six months after the commencement date of sales in the IPO and expiring on the fifth anniversary of the commencement date of sales in the IPO. The selling agent warrants will have a cashless exercise provision and will provide for registration rights with respect to the registration of the shares underlying the warrants.
The Company estimates its current cash resources, including the approximately $9.8 million of net proceeds from the IPO is sufficient to fund its operations into but not beyond the second calendar quarter of 2025. The Company recognizes it will need to raise additional capital to continue to execute its business plan, including obtaining regulatory clearance for its products currently under development and commercializing and generating revenues from products under development. There is no assurance that additional financing will be available when needed or that management will be able to obtain financing on terms acceptable to the Company. A failure to raise sufficient capital, generate sufficient product revenues, control expenditures and regulatory matters, among other factors, will adversely impact the Company’s ability to meet its financial obligations as they become due and payable and to achieve its intended business objectives. If the Company is unable to raise sufficient additional funds, it will have to scale back its operations.
Basis of Presentation
Use of Estimates in Financial Statement Presentation
The preparation of these financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. The Company's significant estimates and assumptions include the valuation of equity related instruments, and initial and recurring fair value measurements for the warrant liability. Although the Company believes that its estimates and assumptions are reasonable, they are based upon information available at the time the estimates and assumptions were made. Some of these judgments can be subjective and complex, and, consequently, actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid accounts with original maturities of three months or less at the date of acquisition to be cash equivalents. Periodically, the Company may carry cash balances at financial institutions in excess of the federally insured limit of $250,000. The Company has not experienced losses on these accounts and management believes, based upon the quality of the financial institutions, that the credit risk with regard to these deposits is not significant.
Offering Costs
Offering costs consist of professional costs incurred through the balance sheet date that are direct and incremental related to the Company’s anticipated IPO. These costs, together with the selling agent fees, were reclassified to additional paid-in capital upon completion of the Company’s IPO on January 26, 2024. Costs associated with salaries and other period costs were expensed as incurred.
During the year ended March 31, 2024, the Company paid $1.3 million of offering costs related to its IPO.
Property and Equipment
Convertible Notes
The Company evaluates embedded redemption, conversion and other features within its debt to determine whether any embedded features should be bifurcated from the host instrument and accounted for as a derivative at fair value, with changes in fair value recorded in the statement of operations.
The Company’s debt is carried on the balance sheet on a historical cost basis net of unamortized discounts and premiums because the Company has not elected the fair value option of accounting. Costs associated with acquiring debt, including detachable warrants issued in connection with the financing, are capitalized as a debt discount. The debt discount is presented in the balance sheet as a direct deduction from the carrying amount of the debt liability. The costs are amortized over the estimated contractual life of the related debt instrument using the effective interest method and are included in interest expense in the statement of operations.
If the Company incurs costs associated with its convertible notes, in advance of the receipt of proceeds, the Company will record a deferred asset. Upon receipt of proceeds the Company will reclassify the deferred asset as a direct deduction from the carrying amount, as described above. In addition, since the instruments included a substantive conversion feature as of time of issuance, the issuance of equity securities were accounted for as a contractual conversion with no gain or loss recognized related to the equity securities issued to settle the instrument.
Fair Value of Financial Instruments
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value maximize the use of observable inputs and minimize the use of unobservable inputs. The Company utilizes a three-level valuation hierarchy for disclosures of fair value measurements, defined as follows:
Level 1 – inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level 2 – inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments.
Level 3 – inputs to the valuation methodology are unobservable and significant to the fair value and require significant judgment and estimation.
The carrying value of short-term instruments, including cash, accounts payable, accrued expenses and convertible notes included in long-term debt, approximate fair value due to the relatively short period to maturity for these instruments.
Related Parties
The Company follows ASC 850, Related Party Disclosures, for the identification of related parties and disclosure of related party transactions. See further discussion in the Notes below on this matter.
Income Taxes
The Company uses the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial reporting and the tax basis of reported assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company must then assess the likelihood that the resulting deferred tax assets will be realized. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized. As of March 31, 2024 and March 31, 2023 the Company determined a full valuation allowance was required to offset its deferred tax assets as a result of recurring operating losses.
The Company accounts for uncertain tax positions in accordance with the provisions of ASC 740-10 which prescribes a recognition threshold and measurement attribute for financial statement disclosure of tax positions taken, or expected to be taken, on its tax return. The Company evaluates and records any uncertain tax positions based on the amount that management deems is more likely than not to be sustained upon examination and ultimate settlement with the tax authorities in the tax jurisdictions in which it operates. As of March 31, 2024 and March 31, 2023 the Company had no uncertain tax positions.
Stock-based Compensation
Employee share-based compensation is measured at the grant date, based on the fair value of the award, and is recognized as an expense over the requisite service period. For awards with a performance condition, compensation expense is recognized over the requisite service period if it is probable that the performance condition will be satisfied. For awards to non-employees, the Company recognizes compensation expense in the same manner as if the Company had paid cash for the goods or services. The Company estimates the fair value of options and equity classified warrants granted using an options pricing model. Expense is recognized within general and administrative and research and development expenses and forfeitures are recognized as they are incurred.
Warrants
The Company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance in FASB ASC 480, Distinguishing Liabilities from Equity (“ASC 480”) and ASC 815, Derivatives and Hedging (“ASC 815”). The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification under ASC 815, including whether the warrants are indexed to the Company’s own ordinary shares and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the Company’s control, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and as of each subsequent quarterly period end date while the warrants are outstanding.
For issued or modified warrants that meet all of the criteria for equity classification, the warrants are required to be recorded as a component of additional paid-in capital at the time of issuance. For issued or modified warrants that do not meet all the criteria for equity classification, the warrants are required to be recorded at their initial fair value on the date of issuance, and each balance sheet date thereafter. Changes in the estimated fair value of the warrants are recognized as a non-cash gain or loss on the statements of operations. The fair value of the warrants is estimated using a Black-Scholes pricing model or a Monte Carlo simulation.
The Company issued warrants to purchase shares of common stock (i) in connection with the Bridge Offering, (ii) as part of selling agent compensation in 2024, and (iii) in connection with the Exclusive License Termination Agreement (the “Termination Agreement”). Based on the guidance noted above, we determined that warrants issued in connection with the Termination Agreement should be accounted for as a liability and the remaining warrants issued meet the requirements for equity classification. Liability classified warrants are subject to remeasurement at each balance sheet date, while equity classified warrants are valued at inception only. As discussed in Note 2, the liability warrants subsequently met equity classification.
Loss Per Common Share
Basic loss per common share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period. Diluted loss per common share is determined using the weighted-average number of common shares outstanding during the period, adjusted for the dilutive effect of common stock equivalents. In periods when losses are reported, the weighted-average number of common shares outstanding excludes common stock equivalents, because their inclusion would be anti-dilutive. Generally, the Company’s outstanding warrants are non-participating securities as they are not entitled to non-forfeitable rights to dividends or dividend equivalents during the vesting term and have no obligation to fund losses. The dilutive effect of convertible securities is calculated using the “if-converted method.” Under the if-converted method, securities are assumed to be converted at the beginning of the period, and the resulting common shares are included in the denominator of the diluted calculation for the entire period being presented. However, the warrants described in Note 2 are participating securities as they receive a right to dividends, but they are not obligated to fund losses. In periods of loss, since no income is allocated to these securities, the Company's use of the treasury stock method derives the same result.
For the twelve months ended March 31, 2024 and 2023, dilutive securities that were not included in the calculations of the loss per common share because they would be anti-dilutive included the following:
|
March 31, |
||||||||
|
2024 |
2023 |
|||||||
|
Equity based warrants to purchase common shares |
5,744,569 | 6,569,929 | ||||||
|
Convertible Notes - common shares (1) |
665,000 | — | ||||||
|
Convertible Notes - equity-based warrants to purchase common shares |
500,000 | — | ||||||
|
Stock options granted under Company's incentive plan |
2,003,600 | — | ||||||
|
Total potentially dilutive securities |
8,913,169 | 6,569,929 | ||||||
|
(1) |
Shares relating to the conversion of the convertible notes as of March 31, 2024 |
Research and Development Costs
Research and development costs are expensed as incurred.
Advertising
It is our policy to expense advertising costs as incurred. Advertising expenses are included within general and administrative expenses within the statement of operations. For the years ended March 31, 2024 and 2023, the Company recorded $1.7 million and $0.1 million, respectively.
Fair Value of Common Stock
Prior to establishing a public market for the Company’s common stock, the estimated fair value of the Company’s common stock was determined by the Company’s Board of Directors (the "Board") as of the date of each option grant, with input from management, considering the Company’s most recently available third-party valuations of common stock, recent sales of common stock to third parties, and the Company’s board of directors’ assessment of additional objective and subjective factors that it believed were relevant and which may have changed from the date of the most recent valuation through the date of the grant.
JOBS Act Accounting Election
The Company qualifies as an emerging growth company ("EGC"), as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). The JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which is neither an early-stage company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Segments
The Company currently operates in one reportable segment based on management’s view of its business for purposes of evaluating performance and making operating decisions. Based upon this business model, the Company’s Chief Executive Officer, whom the Company has determined to be its chief operating decision-maker, reviews financial information as one operating segment.
Recent Accounting Pronouncements
In December 2023, the FASB issued ASU 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures,” which requires disaggregated information about a reporting entity’s effective tax rate reconciliation as well as information on income taxes paid. The guidance is effective for the Company’s fiscal years beginning after December 15, 2024, with early adoption permitted. The Company does not expect the adoption of this standard to have any material impact on its financial statements.
In June 2016, the FASB issued ASU No. 2016-13, “Financial Instruments – Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments,” which introduced a new model for recognizing credit losses on financial instruments based on an estimate of current expected credit losses (“CECL”). The new guidance applies to loans, accounts receivable, trade receivables, other financial assets measured at amortized cost, loan commitments and other off-balance sheet credit exposures. The new guidance also applies to debt securities and other financial assets measured at fair value through other comprehensive income. Estimated credit losses under CECL consider relevant information about past events, current conditions and reasonable and supporting forecasts that affect the collectability of financial assets. Given the non-revenue nature of the Company, the adoption of this standard did not result in an adoption adjustment or material impact.
The Company does not believe that any recently issued effective pronouncements, or pronouncements issued but not yet effective, if adopted, would have a material effect on the accompanying financial statements.
Reclassifications
Certain prior year amounts have been reclassified for consistency with the current year presentation. These reclassifications had no effect on the reported results of operations.
Correction of an Immaterial Error in the Prior Period Financial Statements
During the fourth quarter of 2024 (March 31, 2024), the Company determined that the prior year financial statements had an error caused by an immaterial classification error of certain research and development expense in accordance with ASC 730. As a result, certain prior year amounts have been revised for consistency with the current year presentation. The Company assessed the materiality of this change in presentation on prior period financial statements in accordance with SEC Staff Accounting Bulletin No. 99, “Materiality,” (ASC Topic 250, Accounting Changes and Error Corrections). Based on this assessment, the Company concluded that these classification error corrections in its Statements of Operations are not material to any previously presented financial statements based upon overall considerations of both quantitative and qualitative factors. The corrections had no impact on the fiscal year 2023 Balance Sheet, Statements of Cash Flows, or Statement of Changes in Stockholders’ Equity. Further, the immaterial corrections did not result in a change in operating losses, net loss, or basic or diluted earnings per share in the Income Statement. Accordingly, the Company corrected the previously reported immaterial errors for the year ended March 31, 2023 in this Annual Report on Form 10-K.
A summary of immaterial corrections reflecting the prior period impact to the Company’s Statement of Operations, for the year ended March 31, 2023 is shown below (in thousands):
|
As Revised |
||||||||||||
|
March 31, 2023 |
Correction |
March 31, 2023 |
||||||||||
|
General and administrative expense |
$ | 1,333 | $ | (88 | ) | $ | 1,245 | |||||
|
Research and development expense |
657 | 88 | 745 | |||||||||
|
Net Loss |
$ | 1,990 | $ | - | $ | 1,990 | ||||||
Note 2 – Warrant Liability and Fair Value of Financial Instruments
Financial assets and financial liabilities are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. While the Company believes that its valuation methods are appropriate, the Company recognizes that the use of different methodologies or assumptions to determine the fair value could result in a different estimate of fair value at the reporting date. The primary assumptions that would significantly affect the fair values are the probability weighting of the different settlement outcomes used.
The Company did not have any assets or liabilities measured at fair value as of or during the 12-month period ending March 31, 2023. There were not any transfers into or out of Level 3 as of March 31, 2024 and March 31, 2023.
The following table summarizes the activity of the Level 3 fair value measurements (in thousands):
|
Warrant Liabilities |
||||
|
Balance as of March 31, 2023 |
$ | — | ||
|
Additions |
4,556 | |||
|
Change in fair value measurements - warrants mark-to-market |
3,444 | |||
|
Settlement and reclassification to equity |
(8,000 | ) | ||
|
Balance as of March 31, 2024 |
$ | — | ||
The Company recognized the initial warrant expense as a component of operating expenses on the statement of operations under warrant expense – termination agreement for $4.6 million and the changes in the fair value under warrant liability – mark-to-market for $3.4 million. There were no changes to the valuation approaches or techniques used for Level 3 measurements.
Warrant Liabilities
As more fully detailed in Note 6 – Related Party Transactions, on July 7, 2023, the Company entered into an Exclusive License Termination Agreement (the “Termination Agreement”) with a licensee in exchange for the issuance, upon the closing of the Company’s IPO within one year of the agreement’s execution, of a warrant (the "Warrant") to purchase shares of the Company for a variable number of shares.
The fair value of the warrant liability has been estimated using a discounted cash flow model under various scenarios and used the probability-weighted expected return method (“PWERM”) comparing the probabilities of different outcomes. The outcomes considered included (i) the closing of a qualified financing as part of the Company’s IPO at various points in time and (ii) the possibility of default whereby the licensee receives nothing. Key assumptions for the model were as follows for the initial measurement:
|
Discount rate at issuance (1) |
20.00% |
|
|
Probability (2) |
70% - 10% - 20% |
|
|
Payment (3) |
$0 - $8,000,000 |
|
|
Expected term (in years) |
0.48 - 0.98 |
| (1) |
The initial discount rate was chosen based on private equity rates of return as described in the AICPA Practice Aid on Valuation of Privately-Held-Company Equity securities issued as compensation. For the recurring fair value measurement, the Company updated the discount rate based upon yield curves estimated to be similar in credit quality to the Company; |
| (2) |
Scenario probability as of issuance was based on timing expectations of management that a qualified offering occurring as of December 31, 2023 was estimated at 70%, respectively; a qualified offering occurring as of June 30, 2024 was estimated at 10%; and no qualified offering occurring was estimated at 20%; |
| (3) |
The warrant has a $0.01 strike price, however, the strike price is low relative to the stock price, making the warrant value close to the value of a stock unit. The agreement has a fixed payment value of $8.0 million, see Note 6 – Related Party Transactions. |
On January 29, 2024, the Company issued 1.6 million warrant shares pursuant to the Termination Agreement.
Note 3 – Convertible Notes Payable
On September 9, 2023, the Company's Board authorized an offering up to $2.0 million in unsecured, non-interest bearing convertible promissory notes (the “Notes”) and accompanying warrants (the “Bridge Financing Warrants”) (collectively, the “Bridge Offering”) that will mature on December 31, 2025. The Notes provided that, on the closing date of the IPO, the outstanding principal would be automatically converted into common stock at the conversion price of $2.00. Each dollar in principal amount of Notes purchased were accompanied by a five-year Bridge Financing Warrant to purchase 0.25 shares of Common stock with an exercise price of $1.00 per share. The Company records the Bridge Financing Warrants as a discount to the Notes.
The Bridge Financing Warrants can be exercised from the date of Notes issuance through the five-year anniversary of the issuance of the Notes. The shares issuable pursuant to the Notes and Bridge Financing Warrants have a 180-day lock-up after the Company’s IPO. Thereafter, the foregoing lock-up agreement will cease to apply to 25% of the purchased shares each month for a period of four months. The Note holders are not permitted to convert their Notes when the holders or any of their affiliates would beneficially own in excess of 4.99% of the Company’s common stock after such conversion.
As of March 31, 2024, the Company received proceeds of $2.0 million of Notes executed from the Bridge Offering. Upon the closing of the IPO, certain notes were to be automatically converted according to their terms into the Company’s common stock to the extent and provided that certain holders of these notes are not permitted to convert such notes to the extent that the holders or any of its affiliates would beneficially own in excess of 4.99% of the Company’s common stock after such conversion. Due to this 4.99% limitation, principal representing $1.3 million, or 665,000 shares, of these notes remained outstanding. As discussed in Note 1, the remaining notes converted into common stock in accordance with their original terms on the IPO.
The table below summarizes the Company’s outstanding convertible notes payable as of March 31, 2024 (in thousands).
|
Principal Amount |
Amortized Debt Discount |
Net Carrying Amount |
||||||||||
|
Zero-coupon convertible notes payable due on December 31, 2025 |
$ | 1,330 | $ | 328 | $ | 1,002 | ||||||
Warrants
The Company issued the Notes with detachable warrants for the purchase of shares of the Company’s common stock. The Company utilized a Monte Carlo simulation model to determine the fair value of each Bridge Offering Warrant. During the year ended March 31, 2024, the Company issued warrants valued at $0.6 million. The key inputs to the Monte Carlo simulation used to determine the fair value of each warrant include, the Company’s stock price fair value which was determined through a back solve calculation such that the stock price results in the average total value of the Notes and the Bridge Offering Warrants being equal to the cash proceeds received, volatility based on a selection of publicly held peer companies of 101.88%, expected term of 5 years, risk free rate of 4.40%, discount rate of 20.00% and a discount for lack of marketability of 15.77%.
During the year ended March 31, 2024, the Company recorded less than $0.1 million in interest expense related to the amortization of the debt discount.
The following table presents a summary of activity for the warrants issued in connection with the Company’s Notes:
|
Warrants |
Weighted-Average Exercise Price Per Share |
Remaining Life (In Years) |
Aggregate Intrinsic Value* |
|||||||||||||
|
Outstanding and exercisable, March 31, 2023 |
— | $ | — | — | $ | — | ||||||||||
|
Granted |
500,000 | 1.00 | — | — | ||||||||||||
|
Exercised |
— | — | — | — | ||||||||||||
|
Forfeited/Cancelled |
— | — | — | — | ||||||||||||
|
Expired |
— | — | — | — | ||||||||||||
|
Outstanding, March 31, 2024 |
500,000 | $ | 1.00 | 4.48 | $ | 1,010,000 | ||||||||||
|
Exercisable, March 31, 2024 |
500,000 | $ | 1.00 | 4.48 | $ | 1,010,000 | ||||||||||
*Aggregate Intrinsic Value = Excess of market value over the exercise price of all in-the-money warrants.
Note 4 – Equity
On November 29, 2023, the Company’s Board of Directors and applicable shareholders approved to amend and restate the Company’s certificate of incorporation and increased the authorized shares to 500,000,000 shares of common stock, with a par value of $.001 per share, and 10,000,000 shares of preferred stock, with a par value of $.001 per share. The specific rights of the preferred stock shall be determined by the Board of Directors.
Preferred Stock
As of March 31, 2024, the Company had no shares of preferred stock outstanding.
Restricted Stock
On February 15, 2024, the Company issued 35,000 restricted shares of common stock to the Company's marketing consultant at the closing price of $3.80 of the Company's common stock. The total value of these shares is $133,000. These shares vest monthly over a 12-month period beginning on the issue date.
|
Year ended March 31, |
||||||||
|
2024 |
2023 |
|||||||
|
Recognized in general and administrative expense |
$ | 16,625 | $ | — | ||||
|
Total |
$ | 16,625 | $ | — | ||||
For the year ended March 31, 2024, there was $116,375 of unrecognized stock-based compensation expense related to unvested Restricted Stock, which is expected to be recognized over the period April 2024 through February 2025.
A summary of activity regarding Restricted Stock issued is as follows:
|
Grant Date |
||||||||
|
Number of Shares |
Fair Value Per Share |
|||||||
|
Outstanding, March 31, 2023 |
— | $ | — | |||||
|
Granted |
35,000 | $ | 3.80 | |||||
|
Vested |
(2,917 | ) | $ | 3.80 | ||||
|
Unvested, March 31, 2024 |
32,083 | $ | 3.80 | |||||
Common Stock
On April 6, 2023, the Board of Directors approved a private placement offering of up to 2,000,000 common shares at a price of $2.00 per share. During the year ended March 31, 2024, the Company sold 1,420,000 shares for cash proceeds of $2,840,000. The Company did not incur any costs that were direct and incremental to the private placement.
On September 9, 2023, the Board approved a Bridge Offering. See Note 3 Convertible Notes Payable for additional detail as these notes are convertible into common stock.
Stock Plan and Stock Options
In June 2023, the Company adopted, and the Company’s shareholders approved, the Autonomix Medical, Inc. 2023 Stock Plan (the “Plan”). The Plan is a stock-based compensation plan that provides for discretionary grants of stock options, stock awards and stock unit awards to key employees, non-employee directors, and consultants, subject to certain individual threshold limitations. The Plan provides for up to 4,000,000 shares to be issued. Shares that are surrendered because of forfeiture, expiration, termination, or cancellation are available for re-issuance. As of March 31, 2024, the Plan has 1,996,400 shares remaining available to be issued.
In August 2023, the Plan was amended to allow for an automatic increase of the available shares for issuance, whereby on the 1st of each fiscal year, beginning on April 1, 2024 and ending on (and including) April 1, 2033 in an amount equal to five percent (5%) of the total number of shares of Common Stock outstanding on the March 31st immediately preceding the applicable date. However, the Board may act prior to the automatic increase of a given year to provide that there will be no increase for such year, or that the increase for such year will be a lesser number of shares of Common Stock. The Board did not take any such action and on April 1, 2024, the increase took place.
The following table summarizes the stock option activity for the year ended March 31, 2024. There were no options outstanding during the year ended March 31, 2023.
|
Options |
Weighted-Average Exercise Price Per Share |
Weighted-Average Remaining Life (In Years) |
Aggregate Intrinsic Value* |
|||||||||||||
|
Outstanding and exercisable, March 31, 2023 |
— | $ | — | — | $ | — | ||||||||||
|
Granted |
2,003,600 | 2.33 | — | — | ||||||||||||
|
Exercised |
— | — | — | — | ||||||||||||
|
Forfeited/Cancelled |
— | — | — | — | ||||||||||||
|
Expired |
— | — | — | — | ||||||||||||
|
Outstanding, March 31, 2024 |
2,003,600 | $ | 2.33 | 9.35 | $ | 1,680,672 | ||||||||||
|
Exercisable, March 31, 2024* |
239,217 | $ | 2.00 | 8.96 | $ | 244,001 | ||||||||||
*Aggregate Intrinsic Value = Excess of market value over the exercise price of all in-the-money stock.
During the year ended March 31, 2024, the Company granted certain individuals options to purchase 2,003,600 shares of common stock with contractual terms ranging from three years to ten years, and vesting periods that included monthly over one year, quarterly over one year, monthly over four years, and annually over four years. The options had an aggregate grant date fair value of $3.7 million that was calculated using the Black-Scholes option pricing model. Variables used in the Black-Scholes option pricing model included the following: (1) fair value of common stock on the measurement date of $2.00 per share for options granted as of September 30, 2023, $5.00 per share for options granted subsequent to September 30, 2023 but prior to our IPO and $2.70 for options granted subsequent to our IPO; (2) discount rate ranging from 4.02% to 4.98% based on the daily yield curve rates for U.S. Treasury obligations, (3) expected life ranging from 1.77 years to 6.25 years based on the simplified method (vesting plus contractual term divided by two), and (4) expected volatility ranging from 95% to 119% based on the historical volatility of comparable companies' stock.
All options issued and outstanding are being amortized over their respective vesting periods. The unrecognized compensation expense at March 31, 2024 was $3.1 million. During the year ended March 31, 2024, the Company recorded stock-based compensation - option expense of $0.6 million, of which $0.5 million was recorded in general and administrative expenses and $0.1 million was recorded in research and development expenses in the statements of operations. There was no recorded stock-based compensation - option expense for the year ended March 31, 2023.
Equity-Based Stock Warrants
On March 26, 2024, the Company issued five-year warrants to the selling agent in the Company's IPO to purchase 59,765 shares of common stock at an exercise price of $6.25. Under the fair value method, the fair value of these warrants was estimated on the grant date using the Black-Scholes option pricing model. Variables used in the Black-Scholes warrant pricing model included the following: (1) fair value of common stock on the measurement date of $5.00 per share; (2) discount rate of 4.04% based on the daily yield curve rates for U.S. Treasury obligations; (3) expected life of 5 years and (4) expected volatility of 104% based on the historical volatility of comparable companies' stock. The costs associated with these shares were reclassified to additional paid-in capital upon completion of the Company’s IPO on January 26, 2024.
The Company will periodically grant warrants to investors in connection with equity financing or to third-party service providers in exchange for services rendered. The following table summarizes the stock warrant activity for the year ended March 31, 2024:
|
Warrants |
Weighted-Average Exercise Price Per Share |
Weighted-Average Remaining Life (In Years) |
Aggregate Intrinsic Value* |
|||||||||||||
|
Outstanding and exercisable, March 31, 2023 |
6,569,929 | $ | 0.02 | 5.99 | $ | 12,982,587 | ||||||||||
|
Granted |
1,679,765 | 0.25 | — | — | ||||||||||||
|
Exercised** |
(2,485,301 | ) | 0.03 | — | — | |||||||||||
|
Forfeited/Cancelled |
(19,824 | ) | 1.50 | — | — | |||||||||||
|
Expired |
— | — | — | — | ||||||||||||
|
Outstanding, March 31, 2024 |
5,744,569 | $ | 0.08 | 4.80 | $ | 17,072,147 | ||||||||||
|
Exercisable, March 31, 2024 |
5,736,236 | $ | 0.08 | 4.80 | $ | 17,063,647 | ||||||||||
*Aggregate Intrinsic Value = Excess of market value over the exercise price of all in-the-money stock.
**All exercised shares utilized the “cashless exercise” option.
The unrecognized compensation expense at March 31, 2024 was less than $0.1 million. During the year ended March 31, 2024, the Company recorded stock-based compensation - warrant expense of less than $0.1 million, respectively. There was no recorded stock-based compensation - warrant expense for the year ended March 31, 2023.
Under the fair value method, the fair value of each warrant was estimated on the grant date using the Black-Scholes option pricing model. Variables used in the Black-Scholes warrant pricing model included the following:
|
Range |
||||||||
|
2023 |
2024 |
|||||||
|
Fair value of common stock on the measurement date (per share) |
— |
$2.00 - to $5.00 |
||||||
|
Discount rate based on the daily yield curve rates for U.S. Treasury obligations |
— | 4.04% to 4.54% | ||||||
|
Expected life |
— |
3 to 5 years |
||||||
|
Expected volatility based on the historical volatility of comparable companies' stock |
— | 104% to 119% | ||||||
Note 5 – Commitments and Contingencies
Legal Proceedings
From time to time, we may be involved in claims that arise during the ordinary course of business. Although the results of litigation and claims cannot be predicted with certainty, we do not currently have any pending litigation to which we are a party or to which our property is subject that we believe to be material. Regardless of the outcome, litigation can be costly and time consuming, and it can divert management’s attention from important business matters and initiatives, negatively impacting our overall operations.
Employment Agreements
The Company has agreements with key employees to provide certain benefits, including salary and other wage-related benefits, in the event of termination. In addition, the Company has adopted a severance policy for certain key members of executive management to provide certain benefits, including salary and other wage-related benefits, in the event of termination without cause. In total, these benefits would amount to $0.8 million using the rate of compensation in effect at March 31, 2024.
Note 6 – Related Party Transactions
The Company utilizes a consulting firm that is owned by the Company’s former Chief Financial Officer to provide accounting and financial reporting services and pays certain expenses on behalf of the Company. During the year ended March 31, 2024 and 2023, the Company incurred fees of less than $0.1 million, respectively, for these services, excluding officer compensation. As of March 31, 2024 and March 31, 2023, the Company owed the consulting firm less than $0.1 million, respectively, for services and expenses.
As of March 31, 2024, members of the Company’s management/Board and an immediate family member of the Company’s management (related party), collectively purchased $0.5 million ($0.4 million and $0.1 million, respectively) of the Bridge Offering.
On December 21, 2021, the Company entered into a perpetual, worldwide, exclusive license agreement (the “License” or “License Agreement”) with a company controlled by a significant stockholder of the Company (the “Licensee”). The License allows the Licensee to use certain intellectual property and technology related to the diagnosis and treatment of cardiovascular conditions held by the Company. Upon 90 days following the completion of an IPO or special purpose acquisition company transaction, the Licensee may enter into sublicenses of the licensed intellectual property and technology.
On July 7, 2023, the Company and the Licensee entered into an Exclusive License Termination Agreement (the “Termination Agreement”) in exchange for the issuance, upon the closing of the Company’s IPO within one year of the agreement’s execution, of a warrant to purchase shares of the Company for a variable number of shares. Upon the Company's closing of its IPO on January 29, 2024, 1.6 million warrant shares were issued at $5.00 per share for a fixed value of $8.0 million. The warrants are exercisable at a price of $0.001 per share and may be exercised any time after the issuance date, subject to a beneficial ownership limitation, and expire five years from the original issuance. The warrants contain dividend rights commensurate with the holders of common stock. The warrants do not include any other stockholder rights or privileges prior to exercise.
Note 7 – Income Taxes
The Company files U.S. federal and various U.S. state income tax returns. Due to the Company’s losses, there was no income tax expense for the years ended March 31, 2024 and 2023 (in thousands):
| March 31, | March 31, | |||||||||||||||
| 2024 | 2023 | |||||||||||||||
|
Amount |
% |
Amount |
% |
|||||||||||||
|
Tax benefit at the U.S. federal statutory rate |
$ | (3,239 | ) | 21.00 | % | $ | (418 | ) | 21.00 | % | ||||||
|
Tax rate change |
— | — | $ | — | — | % | ||||||||||
|
Permanent differences |
1,697 | (11.01 | )% | $ | — | — | % | |||||||||
|
Return to provision |
(69 | ) | 0.45 | % | $ | — | — | % | ||||||||
|
Change in state rate |
(190 | ) | 1.23 | % | $ | — | — | % | ||||||||
|
State tax (net of federal benefit) |
(192 | ) | 1.24 | % | $ | — | — | % | ||||||||
|
Valuation allowance |
1,993 | (12.91 | )% | $ | 418 | (21.00 | )% | |||||||||
|
Effective income tax rate |
$ | — | — | % | $ | — | — | % | ||||||||
The effective income tax rate varied from the statutory rate in 2024 primarily due to permanent differences and the increase in the valuation allowance. The effective income tax rate varied from the statutory rate in 2023 as a result of the increase in the valuation allowance.
Deferred tax assets and liabilities consist of the following (in thousands):
|
March 31, |
March 31, |
|||||||
|
2024 |
2023 |
|||||||
|
Assets related to: |
||||||||
|
Capitalized R&D costs |
$ | 602 | $ | 124 | ||||
|
Net operating losses |
2,643 | 1,342 | ||||||
|
Accrual to cash |
72 | — | ||||||
|
Stock-based compensation |
142 | — | ||||||
|
Total deferred tax assets |
3,459 | 1,466 | ||||||
|
Valuation allowance for deferred tax assets |
(3,459 | ) | (1,466 | ) | ||||
|
Net deferred tax |
— | — | ||||||
|
Net deferred tax assets |
$ | — | $ | — | ||||
At March 31, 2024, the Company had U.S. federal net operating loss ("NOL") carry forwards of $11.2 million. Approximately $3.4 million of the U.S. federal NOLs will start expiring in 2034. Additionally, the Company generated a U.S. federal NOL carry forward of approximately $7.7 million post-2017 to 2024. Under the new Tax Act, post-2017 federal NOL carry forwards do not expire, but can only offset 80% of taxable income in the year the loss carry forward is used. The Company also had state NOL carry forwards of approximately $11.1 million which begin to expire in 2034.
Sections 382 and 383 of the Internal Revenue Code limit the annual use of NOL carry forwards and tax credit carry forwards, respectively, following an ownership change. NOL carry forwards may be subject to annual limitations under Internal Revenue Code Section 382 (Section 382) (or comparable provisions of state law) if certain changes in ownership were to occur. The Company is pre-revenue and has been generating net operating losses. Therefore, no NOLs are being utilized nor subject to any utilization limitations. Determination of ownership change or limitation hasn’t been calculated; however, the Company will perform the NOL limitation analysis under Section 382 before any NOLs are expected to be utilized.
The Company has recorded a full valuation allowance against its net total deferred tax assets as of March 31, 2024 and 2023 because management determined that it is not more-likely-than not that those assets will be realized. In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some portion or all of deferred assets will not be realized. The ultimate realization of the deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. During the year ended March 31, 2024, the valuation allowance increased by $1.9 million mainly due to additional net operating losses and capitalized R&D Costs.
The Company is subject to income taxes in the U.S. federal jurisdiction, and various state jurisdictions. Tax regulations within each jurisdiction are subject to the interpretation of the related tax laws and regulations and require significant judgment to apply. As of March 31, 2024, all of the tax years remained open to examination by the federal and state taxing authorities, for three or four years from the tax year in which net operating losses or tax credits are utilized completely.
As of March 31, 2024, the Company has no reserve for uncertain tax positions.
Note 8 – Subsequent Events
On April 1, 2024, pursuant to the provisions of the Company's 2023 Stock Plan, the shares of Common Stock underlying the Plan increased by five percent (5%) of the total number of shares of Common Stock outstanding on the March 31st immediately preceding the applicable date. This resulted in increasing the available shares of Common Stock under the Plan by 942,305.
On April 5, 2024, the Company granted 75,000 stock options to a new employee. These stock options vest in four equal annual installments.
Autonomix Medical, Inc.
Condensed Balance Sheets
(Unaudited)
|
(in thousands, except share and per share data) |
As of |
|||||||
|
June 30, |
March 31, |
|||||||
|
2024 |
2024 |
|||||||
|
Assets |
||||||||
|
Current assets: |
||||||||
|
Cash and cash equivalents |
$ | 6,751 | $ | 8,608 | ||||
|
Other current assets |
325 | 783 | ||||||
|
Total current assets |
7,076 | 9,391 | ||||||
|
Long term assets: |
||||||||
|
Fixed assets, net |
19 | 16 | ||||||
|
Total long term assets |
19 | 16 | ||||||
|
Total Assets |
$ | 7,095 | $ | 9,407 | ||||
|
Liabilities and Stockholders' Equity |
||||||||
|
Current liabilities: |
||||||||
|
Accounts payable |
$ | 287 | $ | 492 | ||||
|
Accrued expenses |
476 | 285 | ||||||
|
Total current liabilities |
763 | 777 | ||||||
|
Long term liabilities: |
||||||||
|
Long term debt - convertible notes, net of unamortized debt discount |
1,043 | 1,002 | ||||||
|
Total long term liabilities |
1,043 | 1,002 | ||||||
|
Total Liabilities |
$ | 1,806 | $ | 1,779 | ||||
|
Commitments and contingencies (Note 5) |
||||||||
|
Stockholders' equity: |
||||||||
|
Preferred stock, $0.001 par value, 10,000,000 shares authorized as of June 30, 2024 and March 31, 2024, respectively, no shares issued and outstanding as of June 30, 2024 and March 31, 2024, respectively |
$ | - | $ | - | ||||
|
Common stock, $0.001 par value, 500,000,000 shares authorized as of June 30, 2024 and March 31, 2024, respectively, 19,242,081 and 18,846,094 shares issued and outstanding as of June 30, 2024 and March 31, 2024, respectively |
19 | 19 | ||||||
|
Additional paid-in capital |
46,938 | 46,578 | ||||||
|
Accumulated deficit |
(41,668 | ) | (38,969 | ) | ||||
|
Total Stockholders' Equity |
5,289 | 7,628 | ||||||
|
Total Liabilities and Stockholders' Equity |
$ | 7,095 | $ | 9,407 | ||||
See accompanying notes to the unaudited condensed financial statements.
Autonomix Medical, Inc.
Condensed Statements of Operations
(Unaudited)
|
Three Months Ended |
||||||||
|
June 30, |
||||||||
|
(in thousands, except share and per share data) |
2024 |
2023 |
||||||
|
Operating expenses: |
||||||||
|
General and administrative |
$ | 1,799 | $ | 503 | ||||
|
Research and development |
954 | 368 | ||||||
|
Total operating expenses |
2,753 | 871 | ||||||
|
Loss from operations |
(2,753 | ) | (871 | ) | ||||
|
Other (expense) income: |
||||||||
|
Interest expense |
(41 | ) | - | |||||
|
Interest income |
95 | 6 | ||||||
|
Total other income |
54 | 6 | ||||||
|
Loss before income taxes |
(2,699 | ) | (865 | ) | ||||
|
Income taxes |
- | - | ||||||
|
Net loss |
$ | (2,699 | ) | $ | (865 | ) | ||
|
Loss per share - basic and diluted |
$ | (0.14 | ) | $ | (0.07 | ) | ||
|
Weighted average shares outstanding - basic and diluted |
18,902,248 | 12,884,604 | ||||||
See accompanying notes to the unaudited condensed financial statements.
Autonomix Medical, Inc.
Condensed Statements of Changes in Stockholders' Equity
(Unaudited)
|
Additional |
Total |
|||||||||||||||||||||||||||
|
Preferred Stock |
Common Stock |
Paid-in |
Accumulated |
Stockholders' |
||||||||||||||||||||||||
|
(in thousands) |
Shares |
Amount |
Shares |
Amount |
Capital |
Deficit |
Equity |
|||||||||||||||||||||
|
Balance March 31, 2023 |
- | - | 12,337 | 12 | 24,175 | (23,543 | ) | 644 | ||||||||||||||||||||
|
Net loss |
- | - | - | - | - | (865 | ) | (865 | ) | |||||||||||||||||||
|
Issuance of common stock |
- | - | 1,420 | 2 | 2,838 | - | 2,840 | |||||||||||||||||||||
|
Balance June 30, 2023 |
- | - | 13,757 | 14 | 27,013 | (24,408 | ) | 2,619 | ||||||||||||||||||||
|
Balance March 31, 2024 |
- | - | 18,846 | 19 | 46,578 | (38,969 | ) | 7,628 | ||||||||||||||||||||
| - | - | |||||||||||||||||||||||||||
|
Net loss |
- | - | - | - | - | (2,699 | ) | (2,699 | ) | |||||||||||||||||||
|
Stock-based compensation |
- | - | - | - | 360 | - | 360 | |||||||||||||||||||||
|
Refund of common stock from IPO |
- | - | (1 | ) | - | - | - | - | ||||||||||||||||||||
|
Issuance of common stock - warrants exercised |
- | - | 397 | - | - | - | - | |||||||||||||||||||||
|
Balance June 30, 2024 |
- | - | 19,242 | 19 | 46,938 | (41,668 | ) | 5,289 | ||||||||||||||||||||
See accompanying notes to the unaudited condensed financial statements.
Autonomix Medical, Inc.
Condensed Statements of Cash Flows
(Unaudited)
|
Three Months Ended June 30, |
||||||||
|
(in thousands) |
2024 |
2023 |
||||||
|
Cash Flows from Operating Activities: |
||||||||
|
Net loss |
$ | (2,699 | ) | $ | (865 | ) | ||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
|
Stock-based compensation |
360 | - | ||||||
|
Depreciation and amortization expense |
43 | - | ||||||
|
Changes in operating assets - decrease/(increase): |
||||||||
|
Other current assets |
458 | (25 | ) | |||||
|
Changes in operating liabilities - (decrease)/increase: |
||||||||
|
Accounts payable |
(205 | ) | 209 | |||||
|
Accrued expenses |
191 | 20 | ||||||
|
Net cash used in operating activities |
(1,852 | ) | (661 | ) | ||||
|
Cash Flows from Investing Activities: |
||||||||
|
Purchase of property and equipment |
(5 | ) | - | |||||
|
Net cash used in investing activities |
(5 | ) | - | |||||
|
Cash Flows from Financing Activities: |
||||||||
|
Issuance of common stock |
- | 2,840 | ||||||
|
Payment of offering costs |
- | (105 | ) | |||||
|
Net cash provided by financing activities |
- | 2,735 | ||||||
|
Net change in cash and cash equivalents |
(1,857 | ) | 2,074 | |||||
|
Cash and cash equivalents, at beginning of period |
8,608 | 865 | ||||||
|
Cash and cash equivalents, at end of period |
$ | 6,751 | $ | 2,939 | ||||
|
Supplemental cash flow disclosures: |
||||||||
|
Non-cash financing activities: |
||||||||
|
Cashless exercise of warrants |
$ | 4 | $ | - | ||||
See accompanying notes to the unaudited condensed financial statements.
Autonomix Medical, Inc.
Notes to the Unaudited Condensed Financial Statements
Note 1 – Description of the Business, Basis of Presentation and Summary of Significant Accounting Policies
Description of the Business
Autonomix Medical, Inc. (“we,” “our,” the “Company”) is a medical device company organized as a Delaware corporation on June 10, 2014. The Company is a pre-revenue, clinical stage life sciences company focused on advancing innovative technologies for sensing and treating disorders relating to the peripheral nervous system.
Liquidity and Going Concern
The Company's financial statements are prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The Company is an early-stage company that is subject to all the risks associated with early-stage and emerging growth companies and has incurred losses since inception.
For the three months ended June 30, 2024 and 2023, the Company incurred net losses of $2.7 million and $0.9 million, respectively, and had net cash flows used in operating activities of $1.9 million and $0.7 million, respectively. The Company had no revenues for the three months ended June 30, 2024 and 2023, respectively, accumulated deficit of $41.7 million, working capital of $6.3 million and cash of $6.8 million. The Company does not expect to generate positive cash flows from operating activities in the near future.
The Company estimates its current cash resources is sufficient to fund its operations into but not beyond the second calendar quarter of 2025. The Company recognizes it will need to raise additional capital to continue to execute its business plan, including obtaining regulatory clearance for its products currently under development and commercializing and generating revenues from products under development. There is no assurance that additional financing will be available when needed or that management will be able to obtain financing on terms acceptable to the Company. A failure to raise sufficient capital, generate sufficient product revenues, control expenditures and regulatory matters, among other factors, will adversely impact the Company’s ability to meet its financial obligations as they become due and payable and to achieve its intended business objectives. If the Company is unable to raise sufficient additional funds, it will have to scale back its operations.
These factors raise substantial doubt about the Company's ability to continue as a going concern within one year after the date the financial statements are issued. The accompanying condensed financial statements have been prepared on a going concern basis and do not include any adjustments that might be necessary if the Company is unable to continue as a going concern.
Basis of Presentation
The accompanying condensed interim financial statements are unaudited. These unaudited condensed interim financial statements have been prepared in accordance with the rules and regulations of the U.S. Securities and Exchange Commission (“SEC”) for interim financial information. Accordingly, they do not include all the information and notes required by generally accepted accounting principles in the United States of America (“GAAP”) for complete financial statements. The Company’s fiscal year end is March 31st. These unaudited condensed interim financial statements should be read in conjunction with the audited financial statements and accompanying notes for the year ended March 31, 2024 as found in the Annual Report in our Form 10-K filed with the SEC on May 31, 2024. In the opinion of management, the unaudited condensed interim financial statements reflect all the adjustments (consisting of normal recurring adjustments) necessary to state fairly the Company’s financial position, results of operations and cash flows for the quarterly and year-to-date periods, as applicable. The interim results of operations are not necessarily indicative of the results that may occur for the full fiscal year. The March 31, 2024 audited condensed balance sheet included herein was derived from the audited financial statements, but does not include all disclosures, including notes, required by GAAP for complete financial statements.
Use of Estimates in Financial Statement Presentation
The preparation of these unaudited condensed interim financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. The Company's significant estimates and assumptions include work performed but not yet billed by contract manufacturers, engineers and research organizations and the valuation of equity related instruments. Although the Company believes that its estimates and assumptions are reasonable, they are based upon information available at the time the estimates and assumptions were made. Some of these judgments can be subjective and complex, and, consequently, actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid accounts with original maturities of three months or less at the date of acquisition to be cash equivalents. Periodically, the Company may carry cash balances at financial institutions in excess of the federally insured limit of $250,000. The Company has not experienced losses on these accounts and management believes, based upon the quality of the financial institutions, that the credit risk with regard to these deposits is not significant.
Offering Costs
Offering costs consist of professional costs incurred through the balance sheet date that are direct and incremental related to the Company’s initial public offering ("IPO"). These costs, together with the selling agent fees, were reclassified to additional paid-in capital upon completion of the Company’s IPO on January 26, 2024. Costs associated with salaries and other period costs were expensed as incurred.
During the three months ended June 30, 2024 and 2023, the Company paid zero and $0.1 million, respectively, of offering costs related to its IPO.
Property and Equipment
Property and equipment (comprised of computer and IT equipment) are stated at historical cost and depreciated on a straight-line basis over their estimated useful lives, generally three years. Upon disposition of the assets, the costs and related accumulated depreciation are removed from the accounts and any resulting gain or loss is included in the results of operations.
Convertible Notes
The Company evaluates embedded redemption, conversion and other features within its debt to determine whether any embedded features should be bifurcated from the host instrument and accounted for as a derivative at fair value, with changes in fair value recorded in the condensed statements of operations.
The Company’s debt is carried on the condensed balance sheets on a historical cost basis net of unamortized discounts and premiums because the Company has not elected the fair value option of accounting. Costs associated with acquiring debt, including detachable warrants issued in connection with the financing, are capitalized as a debt discount. The debt discount is presented in the condensed balance sheets as a direct deduction from the carrying amount of the debt liability. The costs are amortized over the estimated contractual life of the related debt instrument using the effective interest method and are included in interest expense in the condensed statements of operations.
If the Company incurs costs associated with its convertible notes, in advance of the receipt of proceeds, the Company will record a deferred asset. Upon receipt of proceeds the Company will reclassify the deferred asset as a direct deduction from the carrying amount, as described above.
In addition, since the instruments included a substantive conversion feature as of time of issuance, the issuance of equity securities to settle the outstanding notes with the conversion were accounted for as a contractual conversion with no gain or loss recognized related to the equity securities issued to settle the instrument.
Fair Value of Financial Instruments
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value maximize the use of observable inputs and minimize the use of unobservable inputs. The Company utilizes a three-level valuation hierarchy for disclosures of fair value measurements, defined as follows:
Level 1 – inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active market
Level 2 – inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instrument
Level 3 – inputs to the valuation methodology are unobservable and significant to the fair value and require significant judgment and estimation.
Financial assets and financial liabilities are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. While the Company believes that its valuation methods are appropriate, the Company recognizes that the use of different methodologies or assumptions to determine the fair value could result in a different estimate of fair value at the reporting date. The primary assumptions that would significantly affect the fair values are the probability weighting of the different settlement outcomes used.
The Company did not have any assets or liabilities measured at fair value as of or during the three-month period ending June 30, 2024 and March 31, 2024. There were not any transfers into or out of Level 3 as of June 30, 2024 and March 31, 2024.
As of June 30, 2024, the Company determined that the estimated fair value of debt was approximately $1.0 million. The fair value of debt was estimated using market rates the Company believes would be available for similar types of financial instruments and represents a Level 2 measurement.
The carrying value of short-term instruments, including cash, accounts payable and accrued expenses, approximate fair value due to the relatively short period to maturity for these instruments.
Related Parties
The Company follows Accounting Standards Codification ("ASC") 850, Related Party Disclosures, for the identification of related parties and disclosure of related party transactions. See further discussion in Note 5 below on this matter.
Income Taxes
The Company uses the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial reporting and the tax basis of reported assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company must then assess the likelihood that the resulting deferred tax assets will be realized. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized. As of June 30, 2024 and March 31, 2024 the Company determined a full valuation allowance was required to offset its deferred tax assets as a result of recurring operating losses.
The Company accounts for uncertain tax positions in accordance with the provisions of ASC 740-10 which prescribes a recognition threshold and measurement attribute for financial statement disclosure of tax positions taken, or expected to be taken, on its tax return. The Company evaluates and records any uncertain tax positions based on the amount that management deems is more likely than not to be sustained upon examination and ultimate settlement with the tax authorities in the tax jurisdictions in which it operates. As of June 30, 2024 and March 31, 2024 the Company had no uncertain tax positions.
The Company does not expect to pay any significant federal, state, or foreign income taxes in our fiscal year 2025 (ending March 31, 2025) as a result of the losses recorded during the three months ended June 30, 2024 and the additional losses expected for the remainder of our fiscal year 2025 and cumulative net operating loss carryforwards. Accounting standards require the consideration of a valuation allowance for deferred tax assets if it is “more likely than not” that some component or all of the benefits of deferred tax assets will not be realized.
The Company recorded no income tax provision for the three months ended June 30, 2024 and 2023, respectively. The effective tax rate for the three months ended June 30, 2024 and 2023 is zero. The Company estimates its annual effective tax rate at the end of each quarterly period. Jurisdictions with a projected loss for the year where no tax benefit can be recognized due to the valuation allowance could result in a higher or lower effective tax rate during a particular quarter depending on the mix and timing of actual earnings versus annual projections.
Stock-based Compensation
Employee and non-employee share-based compensation is measured at the grant date, based on the fair value of the award, and is recognized as an expense over the requisite service period. For awards with a performance condition, compensation expense is recognized over the requisite service period if it is probable that the performance condition will be satisfied. For awards to non-employees, the Company recognizes compensation expense in the same manner as if the Company had paid cash for the goods or services. The Company estimates the fair value of options and equity classified warrants granted using an options pricing model. Expense is recognized within general and administrative expenses and forfeitures are recognized as they are incurred.
Warrants
The Company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance in FASB ASC 480, Distinguishing Liabilities from Equity (“ASC 480”) and ASC 815, Derivatives and Hedging (“ASC 815”). The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification under ASC 815, including whether the warrants are indexed to the Company’s own ordinary shares and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the Company’s control, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and as of each subsequent quarterly period end date while the warrants are outstanding.
For issued or modified warrants that meet all of the criteria for equity classification, the warrants are required to be recorded as a component of additional paid-in capital at the time of issuance. For issued or modified warrants that do not meet all the criteria for equity classification, the warrants are required to be recorded at their initial fair value on the date of issuance, and each balance sheet date thereafter. Changes in the estimated fair value of the warrants are recognized as a non-cash gain or loss on the statements of operations. The fair value of the warrants is estimated using a Black-Scholes pricing model or a Monte Carlo simulation.
Loss Per Common Share
Basic loss per common share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period. Diluted loss per common share is determined using the weighted-average number of common shares outstanding during the period, adjusted for the dilutive effect of common stock equivalents. In periods when losses are reported, the weighted-average number of common shares outstanding excludes common stock equivalents, because their inclusion would be anti-dilutive. Generally, the Company’s outstanding warrants are non-participating securities as they are not entitled to non-forfeitable rights to dividends or dividend equivalents during the vesting term and have no obligation to fund losses.
However, the warrants described in Note 5 are participating securities as they receive a right to dividends, but they are not obligated to fund losses. In periods of loss, since no income is allocated to these securities, the Company's use of the treasury stock method derives the same result. The dilutive effect of convertible securities is calculated using the “if-converted method.” Under the if-converted method, securities are assumed to be converted at the beginning of the period, and the resulting common shares are included in the denominator of the diluted calculation for the entire period being presented.
For the three months ended June 30, 2024 and 2023, dilutive securities that were not included in the calculations of the loss per common share because they would be anti-dilutive included the following:
|
June 30, |
||||||||
|
2024 |
2023 |
|||||||
|
Equity based warrants to purchase common shares |
5,344,569 | 6,569,929 | ||||||
|
Convertible Notes - common shares (1) |
665,000 | - | ||||||
|
Convertible Notes - equity-based warrants to purchase common shares |
500,000 | - | ||||||
|
Stock options granted under Company's incentive plan |
4,329,579 | 933,600 | ||||||
|
Total potentially dilutive securities |
10,839,148 | 7,503,529 | ||||||
Research and Development Costs
Research and development costs are expensed as incurred.
Advertising
It is our policy to expense advertising costs as incurred. Advertising expenses are included within general and administrative expenses within the statement of operations. For the three months ended June 30, 2024 and 2023, the Company recorded less than $0.1 million and $0.1 million, respectively.
Fair Value of Common Stock
Prior to establishing a public market for the Company’s common stock, the estimated fair value of the Company’s common stock was determined by the Company’s board of directors as of the date of each option grant, with input from management, considering the Company’s most recently available third-party valuations of common stock, recent sales of common stock to third parties, and the Company’s board of directors’ assessment of additional objective and subjective factors that it believed were relevant and which may have changed from the date of the most recent valuation through the date of the grant.
JOBS Act Accounting Election
The Company qualifies as an emerging growth company (“EGC”), as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). The JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which is neither an early-stage company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Segments
The Company currently operates in one reportable segment based on management’s view of its business for purposes of evaluating performance and making operating decisions. Based upon this business model, the Company’s Chief Executive Officer, whom the Company has determined to be its chief operating decision-maker, reviews financial information as one operating segment.
Recent Accounting Pronouncements
In December 2023, the FASB issued ASU 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures,” which requires disaggregated information about a reporting entity’s effective tax rate reconciliation as well as information on income taxes paid. The guidance is effective for the Company’s fiscal years beginning after December 15, 2024, with early adoption permitted. The Company does not expect the adoption of this standard to have any material impact on its financial statements.
The Company does not believe that any recently issued effective pronouncements, or pronouncements issued but not yet effective, if adopted, would have a material effect on the accompanying financial statements.
Correction of an Immaterial Error in the Prior Period Financial Statements
During the fourth quarter of fiscal 2024 (March 31, 2024), the Company determined that the prior year financial statements had an error caused by an immaterial classification error of certain research and development expense in accordance with ASC 730 Research and Development Costs. As a result, certain prior year amounts have been revised for consistency with the current year presentation. The Company assessed the materiality of this change in presentation on prior period financial statements in accordance with SEC Staff Accounting Bulletin No. 99, “Materiality,” (ASC Topic 250, Accounting Changes and Error Corrections). Based on this assessment, the Company concluded that these classification error corrections in its Statements of Operations are not material to any previously presented financial statements based upon overall considerations of both quantitative and qualitative factors. The corrections had no impact on the fiscal year 2023 Balance Sheet, Statements of Cash Flows, or Statement of Changes in Stockholders’ Equity. Further, the immaterial corrections did not result in a change in operating losses, net loss, or basic or diluted earnings per share in the Income Statement.
A summary of immaterial corrections reflecting the prior period impact to the Company’s Statement of Operations, for the quarter ended June 30, 2023 is shown below (in thousands):
|
As Revised |
||||||||||||
|
June 30, 2023 |
Correction |
June 30, 2023 |
||||||||||
|
General and administrative expense |
$ | 523 | $ | (20 | ) | $ | 503 | |||||
|
Research and development expense |
348 | 20 | 368 | |||||||||
|
Net Loss |
$ | 871 | $ | - | $ | 871 | ||||||
Note 2 – Convertible Notes Payable
On September 9, 2023, the Company's Board of Directors (the “Board”) authorized an offering up to $2.0 million in unsecured, non-interest bearing convertible promissory notes (the “Notes”) and accompanying warrants (the “Bridge Financing Warrants”) (collectively, the “Bridge Offering”) that will mature on December 31, 2025. The Notes provided that, on the closing date of the IPO, the outstanding principal would be automatically converted into common stock at the conversion price of $2.00. Each dollar in principal amount of Notes purchased were accompanied by a five-year Bridge Financing Warrant to purchase 0.25 shares of Common stock with an exercise price of $1.00 per share. The Company records the Bridge Financing Warrants as a discount to the Notes.
The Bridge Financing Warrants can be exercised from the date of Notes issuance through the five-year anniversary of the issuance of the Notes. The shares issuable pursuant to the Notes and Bridge Financing Warrants have a 180-day lock-up after the Company’s IPO. Thereafter, the foregoing lock-up agreement will cease to apply to 25% of the purchased shares each month for a period of four months. The Note holders are not permitted to convert their Notes when the holders or any of their affiliates would beneficially own in excess of 4.99% of the Company’s common stock after such conversion.
As of June 30, 2024, the Company received proceeds of $2.0 million of Notes executed from the Bridge Offering, which would convert into 1.0 million shares of common stock. The Company’s effective interest rate for the Notes is 15.3% due to the amortization of the discount stemming from the issuance of the Bridge Financing Warrants. On January 26, 2024, we consummated our initial public offering (“IPO”). In connection with the closing of the IPO, a portion of our convertible notes were converted into 335,000 shares of our common stock. Upon the closing of the IPO, certain notes were to be automatically converted according to their terms into our common stock to the extent and provided that certain holders of these notes are not permitted to convert such notes to the extent that the holders or any of its affiliates would beneficially own in excess of 4.99% of our common stock after such conversion. Due to this 4.99% limitation, principal representing $1.3 million, or 665,000 shares, of these notes remains outstanding.
The table below summarizes the Company’s outstanding convertible notes payable as of June 30, 2024 (in thousands).
|
Principal Amount |
Amortized Debt Discount |
Net Carrying Amount |
||||||||||
|
Zero-coupon convertible notes payable due on December 31, 2025 |
$ | 1,330 | $ | 287 | $ | 1,043 | ||||||
Warrants
The Company issued the Notes with detachable warrants for the purchase of shares of the Company’s common stock. The Company utilized a Monte Carlo simulation model to determine the fair value of each Bridge Offering Warrant. The key inputs to the Monte Carlo simulation used to determine the fair value of each warrant include, the Company’s stock price fair value which was determined through a back solve calculation such that the stock price results in the average total value of the Notes and the Bridge Offering Warrants being equal to the cash proceeds received, volatility based on a selection of publicly held peer companies of 101.88%, expected term of 5 years, risk free rate of 4.40%, discount rate of 20.00% and a discount for lack of marketability of 15.77%.
During the three months ended June 30, 2024, the Company recorded less than $0.1 million in interest expense related to the amortization of the debt discount.
The following table presents a summary of activity for the warrants issued in connection with the Company’s Notes:
|
Weighted-Average |
||||||||||||||||
|
Exercise Price |
Remaining Life |
Aggregate |
||||||||||||||
|
Warrants |
Per Share |
(In Years) |
Intrinsic Value* |
|||||||||||||
|
Outstanding and exercisable, March 31, 2024 |
500,000 | $ | 1.00 | 4.48 | $ | 1,010,000 | ||||||||||
|
Granted |
- | - | - | - | ||||||||||||
|
Exercised |
- | - | - | - | ||||||||||||
|
Forfeited/Cancelled |
- | - | - | - | ||||||||||||
|
Expired |
- | - | - | - | ||||||||||||
|
Outstanding, June 30, 2024 |
500,000 | $ | 1.00 | 4.23 | $ | - | ||||||||||
|
Exercisable, June 30, 2024 |
500,000 | $ | 1.00 | 4.23 | $ | - | ||||||||||
*Aggregate Intrinsic Value = Excess of market value over the exercise price of all in-the-money warrants. No outstanding and exercisable warrants issued in connection with the Company's Notes were in-the-money as of June 30, 2024.
Note 3 – Equity
On January 26, 2024, we consummated our IPO. In the IPO, we sold a total of 2,234,222 shares of common stock at a purchase price of $5.00 per share for gross proceeds of $11.2 million and net proceeds of $9.8 million. In connection with the closing of the IPO, a portion of our convertible notes were converted into 335,000 shares of our common stock. Total shares of common stock outstanding at the closing of the IPO amounted to 18,687,061 shares, prior to the May 13, 2024 cancellation of 1,050 shares represented in the IPO for payment disputes. Upon the closing of the IPO, certain notes were to be automatically converted according to their terms into our common stock to the extent and provided that certain holders of these notes are not permitted to convert such notes to the extent that the holders or any of its affiliates would beneficially own in excess of 4.99% of our common stock after such conversion. Due to this 4.99% limitation, principal representing $1.3 million, or 665,000 shares, of these notes remains outstanding.
On November 29, 2023, the Company’s Board of Directors and applicable shareholders approved to amend and restate the Company’s certificate of incorporation and increased the authorized shares to 500,000,000 shares of common stock, with a par value of $.001 per share, and 10,000,000 shares of preferred stock, with a par value of $.001 per share. The specific rights of the preferred stock shall be determined by the Board of Directors.
Restricted Stock
On February 15, 2024, the Company issued 35,000 restricted shares of common stock to the Company's marketing consultant at the closing price of $3.80 of the Company's common stock. The total value of these shares is $133,000. These shares vest monthly over a 12-month period beginning on the issue date.
|
Quarter ended June 30, |
||||||||
|
2024 |
2023 |
|||||||
|
Recognized in general and administrative expense |
$ | 33,250 | $ | — | ||||
|
Total |
$ | 33,250 | $ | — | ||||
For the quarter ended June 30, 2024, there was $83,125 of unrecognized stock-based compensation expense related to unvested Restricted Stock, which is expected to be recognized over the period July 2024 through February 2025.
A summary of activity regarding Restricted Stock issued is as follows:
|
Grant Date |
||||||||
|
Number of Shares |
Fair Value Per Share |
|||||||
|
Unvested, March 31, 2024 |
32,083 | $ | 3.80 | |||||
|
Granted |
— | $ | — | |||||
|
Vested |
(8,750 | ) | $ | 3.80 | ||||
|
Unvested, June 30, 2024 |
23,333 | $ | 3.80 | |||||
Common Stock
On April 6, 2023, the Board of Directors approved a private placement offering of up to 2,000,000 common shares at a price of $2.00 per share. During the three months ended June 30, 2023, the Company sold 1,420,000 shares for cash proceeds of $2,840,000. The Company did not incur any costs that were direct and incremental to the private placement.
On September 9, 2023, the Board approved a Bridge Offering. See Note 3 Convertible Notes Payable for additional detail as these notes are convertible into common stock.
Stock Plan and Stock Options
In June 2023, the Company adopted, and the Company’s shareholders approved, the Autonomix Medical, Inc. 2023 Stock Plan (the “Plan”). The Plan is a stock-based compensation plan that provides for discretionary grants of stock options, stock awards and stock unit awards to key employees, non-employee directors, and consultants, subject to certain individual threshold limitations. The Plan provides for up to 4,000,000 shares to be issued. Shares that are surrendered because of forfeiture, expiration, termination, or cancellation are available for re-issuance.
In August 2023, the Plan was amended to allow for an automatic increase of the available shares for issuance, whereby on the 1st of each fiscal year, beginning on April 1, 2024 and ending on (and including) April 1, 2033 in an amount equal to five percent (5%) of the total number of shares of Common Stock outstanding on the March 31st immediately preceding the applicable date. However, the Board may act prior to the automatic increase of a given year to provide that there will be no increase for such year, or that the increase for such year will be a lesser number of shares of Common Stock. On April 1, 2024, the Plan was increased by 942,305 shares.
The following table summarizes the stock option activity for the three months ended June 30, 2024:
|
Weighted-Average |
Weighted-Average |
|||||||||||||||
|
Exercise Price |
Remaining Life |
Aggregate |
||||||||||||||
|
Options |
Per Share |
(In Years) |
Intrinsic Value* |
|||||||||||||
|
Outstanding and exercisable, March 31, 2024 |
2,003,600 | $ | 2.33 | 9.35 | $ | 1,680,672 | ||||||||||
|
Granted |
2,325,979 | 1.38 | - | - | ||||||||||||
|
Exercised |
- | - | - | - | ||||||||||||
|
Forfeited/Cancelled |
- | - | - | - | ||||||||||||
|
Expired |
- | - | - | - | ||||||||||||
|
Outstanding, June 30, 2024 |
4,329,579 | $ | 1.82 | 9.57 | $ | - | ||||||||||
|
Exercisable, June 30, 2024* |
321,317 | $ | 2.00 | 8.69 | $ | - | ||||||||||
*Aggregate Intrinsic Value = Excess of market value over the exercise price of all in-the-money stock. No outstanding or exercisable options were in-the-money as of June 30, 2024.
During the three months ended June 30, 2024, the Company granted certain individuals options to purchase 2,325,979 shares of common stock with an average exercise price of $1.38 per share and a contractual term that vests annually over four years on the anniversary date. The options had an aggregate grant date fair value of $2.6 million that was calculated using the Black-Scholes option pricing model. Variables used in the Black-Scholes option pricing model included the following: (1) fair value of common stock on the measurement date; (2) discount rate ranging from 4.25% to 4.39% based on the daily yield curve rates for U.S. Treasury obligations, (3) expected life ranging of 6.25 years based on the simplified method (vesting plus contractual term divided by two) and (4) expected volatility ranging from 110% to 130% based on the historical volatility of comparable companies' stock.
All options issued and outstanding are being amortized over their respective vesting periods. The unrecognized compensation expense at June 30, 2024 was $5.4 million. During the three months ended June 30, 2024, the Company recorded stock-based compensation - option expense of $0.3 million in general and administrative expense and less than $0.1 million in research and development expense. There was no recorded stock-based compensation - option expense for the three months ended June 30, 2023.
Equity-Based Stock Warrants
The Company will periodically grant warrants to investors in connection with equity financing or to third-party service providers in exchange for services rendered. The following table summarizes the stock warrant activity for the three months ended June 30, 2024:
|
Weighted-Average |
Weighted-Average |
|||||||||||||||
|
Exercise Price |
Remaining Life |
Aggregate |
||||||||||||||
|
Warrants |
Per Share |
(In Years) |
Intrinsic Value* |
|||||||||||||
|
Outstanding, March 31, 2024 |
5,744,569 | $ | 0.08 | 4.80 | $ | 17,072,147 | ||||||||||
|
Granted |
- | - | - | - | ||||||||||||
|
Exercised** |
(397,037 | ) | 0.01 | - | - | |||||||||||
|
Forfeited/Cancelled |
(2,963 | ) | 0.01 | - | - | |||||||||||
|
Expired |
- | - | - | - | ||||||||||||
|
Outstanding, June 30, 2024 |
5,344,569 | $ | 0.09 | 4.54 | $ | 5,009,401 | ||||||||||
|
Exercisable, June 30, 2024 |
5,341,236 | $ | 0.09 | 4.54 | $ | 5,009,401 | ||||||||||
|
* |
Aggregate Intrinsic Value = Excess of market value over the exercise price of all in-the-money warrants. 5,257,929 outstanding and exercisable warrants were in-the-money as of June 30, 2024. |
|
** |
All exercised warrants utilized the “cashless exercise” option. |
The unrecognized compensation expense at June 30, 2024 was less than $0.1 million. During the three months ended June 30, 2024, the Company recorded stock-based compensation - warrant expense of less than $0.1 million. There was no recorded stock-based compensation - warrant expense for the three months ended June 30, 2023.
Note 4 – Commitments and Contingencies
Legal Proceedings
From time to time, we may be involved in claims that arise during the ordinary course of business. Although the results of litigation and claims cannot be predicted with certainty, we do not currently have any pending litigation to which we are a party or to which our property is subject that we believe to be material. Regardless of the outcome, litigation can be costly and time consuming, and it can divert management’s attention from important business matters and initiatives, negatively impacting our overall operations.
Employment Agreements
We have agreements with key employees to provide certain benefits, including salary and other wage-related benefits, in the event of termination. In addition, the Company has adopted a severance policy for certain key members of executive management to provide certain benefits, including salary and other wage-related benefits, in the event of termination. In total, these benefits would amount to a range of $1.1 million to $1.6 million using the rate of compensation in effect at June 30, 2024.
Brad Hauser - Chief Executive Officer
On June 17, 2024, we entered into an employment agreement with Brad Hauser pursuant to which Mr. Hauser agreed to serve as our chief executive officer and president for an initial three-year period, which may be extended on a year-to-year basis. Mr. Hauser’s agreement provides for an initial annual base salary of $450,000 (subject to an annual review and increase at the discretion of our Compensation Committee) and a target annual bonus of 60% of his base salary. Pursuant to the agreement, Mr. Hauser was granted a ten-year option (the “Inducement Options”) to purchase 900,000 shares of common stock at an exercise price equal to the closing price of our common stock on the date of the employment agreement. The option vests in four equal annual installments (or 225,000 shares each installment) on each of the succeeding four anniversary dates of the execution of the employment agreement, provided Mr. Hauser is employed by us on each vesting date. In the event of a “change of control” or the termination of the agreement by us without “cause” or by Mr. Hauser for “good reason,” all of the unvested options shall immediately vest. The Inducement Options were granted outside of our 2023 Stock Plan as an inducement material to Mr. Hauser’s entering into employment with us in accordance with Nasdaq Stock Market Listing Rule 5635(c)(4). Commencing with the year ending March 31, 2025, Mr. Hauser will be eligible to receive annual option grants as determined by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee. The number of shares underlying the target annual option grant will be equal to $1,000,000 divided by the Black-Scholes value per share of our common stock on the date of grant.
If Mr. Hauser’s employment is terminated at our election without “cause,” or by Mr. Hauser for “good reason,” Mr. Hauser shall be entitled to receive severance payments equal to twelve months of Mr. Hauser’s base salary and 100% of the target bonus for the year in which such termination occurs; provided that such amounts shall be increased by 50% if Mr. Hauser’s agreement is terminated without “cause” or by Mr. Hauser for “good reason” within three months prior to or twelve months after a “change of control.” In the event that any payments or benefits provided to Mr. Hauser would trigger the excise tax under Section 4999 of the Internal Revenue Code or any similar provision, the Company agreed to provide Mr. Hauser with a gross-up payment to ensure that, after payment of all taxes (including the excise tax, federal, state, and local income taxes, and employment taxes) imposed on the gross-up payment, Mr. Hauser receives a net amount equal to the payments or benefits Mr. Hauser would have received if the excise tax didn't apply
Lori Bisson - Vice Chair (former Chief Executive Officer)
On June 17, 2024, we entered into an employment agreement with Lori Bisson pursuant to which Ms. Bisson agreed to serve as our Executive Vice Chair and Strategic Adviser to the Chief Executive Officer (“Vice Chair”) for a two-year period. Ms. Bisson’s agreement provides for an initial annual base salary of $150,000 (subject to an annual review and increase at the discretion of our Compensation Committee) and a target annual bonus of 50% of her base salary. Pursuant to the agreement, Ms. Bisson continued to vest in the option grants issued to Ms. Bisson in her role as chief executive officer and president in accordance with the vesting schedule set out in her initial employment agreement. In the event of a “change of control” or the termination of the agreement by us without “cause” or by Ms. Bisson for “good reason,” all of the unvested options shall immediately vest. Ms. Bisson is entitled to receive any compensation, including incentive compensation, for the fiscal year ended March 31, 2024 that has not been paid as of the date of the agreement. Commencing with the year ending March 31, 2025, Ms. Bisson will be eligible to receive annual option grants as determined by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee. Ms. Bisson agreed to waive any severance payments due to her in connection with the termination of the prior employment agreement that we entered into with her on June 30, 2023.
Note 5 – Related Party Transactions
The Company utilizes a consulting firm that is owned by the Company’s former Chief Financial Officer to provide accounting and financial reporting services and pays certain expenses on behalf of the Company. During the three months ended June 30, 2024 and 2023, the Company incurred fees of $0 and less than $0.1 million, respectively, for these services, excluding officer compensation. As of June 30, 2024 and March 31, 2024, the Company owed the consulting firm $0 and less than $0.1 million, respectively, for services and expenses.
As of June 30, 2024, members of the Company’s management/Board and an immediate family member of the Company’s management (related party), collectively purchased $0.5 million ($0.4 million and $0.1 million, respectively) of the Bridge Offering.
On December 21, 2021, the Company entered into a perpetual, worldwide, exclusive license agreement (the “License” or “License Agreement”) with a company controlled by a significant stockholder of the Company (the “Licensee”). The License allows the Licensee to use certain intellectual property and technology related to the diagnosis and treatment of cardiovascular conditions held by the Company. Upon 90 days following the completion of an initial public offering or special purpose acquisition company transaction, the Licensee may enter into sublicenses of the licensed intellectual property and technology.
On July 7, 2023, the Company and the Licensee entered into an Exclusive License Termination Agreement (the “Termination Agreement”) in exchange for the issuance, upon the closing of the Company’s initial public offering within one year of the agreement’s execution, of a warrant to purchase shares of the Company for a variable number of shares. The variable number of shares issued was based upon a fixed value of $8.0 million divided by the price per share in the offering. The warrants are exercisable at a price of $0.001 per share and may be exercised any time after the issuance date, subject to a beneficial ownership limitation, and expires five years from the original issuance. The warrants provide voting rights, dividend rights, and other rights of a shareholder prior to exercise. The shares underlying the warrant will be subject to a lockup agreement for a period of six months after the closing of the offering with respect to 12.5% of the shares issued and twelve months after the closing of the offering for the remainder of the shares.
On January 29, 2024, we issued a warrant to purchase 1,600,000 shares (the “Warrant”) pursuant to the Termination Agreement with Impulse Medical, Inc. ("Impulse"). The warrants are exercisable at a price of $0.001 per share and may be exercised any time after the issuance date, subject to a beneficial ownership limitation, and expires five years from the original issuance. The warrants provide voting rights, dividend rights, and other rights of a shareholder prior to exercise. The shares underlying the Warrant are subject to a lockup agreement for a period of six months after the closing of the IPO with respect to 12.5% of the shares issued and twelve months after the closing of the IPO for the remainder of the shares. In connection with the Termination Agreement, the Company agreed to register the resale of the shares of common stock underlying the Warrant upon a notice of 20 business days by the Warrant holder.
Note 6 – Subsequent Events
On July 10, 2024, we entered into a license agreement (the “Agreement”) with RF Innovations, Inc. (“RFI”), a privately held medical technology company, to license products utilizing RFI’s intellectual property related to its Apex 6 Radiofrequency Generator (the “Licensed Products”). The Apex 6 Generator is a United States Food and Drug Administration (“FDA”) cleared ablation technology designed to lesion neural tissue for pain management in the peripheral nervous system. Pursuant to the Agreement, RFI granted us a perpetual non-exclusive worldwide royalty free fully paid license related to the Licensed Products, provided that the license did not include the right to sell certain products to customers for the treatment of spine pain. In connection with the Agreement, we issued RFI 250,000 unregistered shares of our common stock as consideration for the license. The Agreement provides RFI the right to terminate the license if we breach any representation, warranty or covenant contained in the Agreement, subject to any relevant cure periods, or if we are subject to a bankruptcy or insolvency event.
In July 2024, 3,594,000 warrants were exercised on a cashless basis resulting in a net share amount of 3,544,852 at an exercise price of $0.01.
Up to 698,812 Common Stock Units
Each Common Stock Unit Consisting of One Share of Common Stock and One Series A Warrant to Purchase One Share of Common Stock
Up to 698,812 Shares of Common Stock Issuable Upon Exercise of Series A Warrants
Up to 698,812 PFW Units
Each PFW Unit Consisting of One Pre-Funded Warrant to Purchase One Share of Common Stock and One Series A Warrant to Purchase One Share of Common Stock
Up to 698,812 Shares of Common Stock Issuable Upon Exercise of Pre-Funded Warrants
Up to 698,812 Shares of Common Stock Underlying the Series A Warrants
Representative Warrants to Purchase up to 41,928 Shares of Common Stock
Up to 41,928 Shares of Common Stock Issuable Upon Exercise of Representative Warrants

Ladenburg Thalmann
PROSPECTUS
________________, 2024
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the estimated costs and expenses to be incurred in connection with the issuance and distribution of the securities of Autonomix Medical, Inc. (the “Registrant”) which are registered under this Registration Statement on Form S-1 (this “Registration Statement”), other than underwriting discounts and commissions. All amounts are estimates except the Securities and Exchange Commission registration fee and the Financial Industry Regulatory Authority, Inc. filing fee.
The following expenses will be borne solely by the Registrant.
|
Amount to be |
||
|
SEC Registration fee |
$ |
3,949.00 |
|
Financial Industry Regulatory Authority, Inc. filing fee |
4,369.00 |
|
|
Legal fees and expenses |
150,000.00 |
|
|
Accounting fees and expenses |
56,000.00 |
|
|
Transfer Agent’s fees |
1,000.00 |
|
|
Miscellaneous fees and expenses |
4,682.00 |
|
|
Total |
$ |
220,000.00 |
Item 14. Indemnification of Directors and Officers.
Pursuant to Section 145 of the Delaware General Corporation Law (the “DGCL”), a corporation shall have the power to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than a derivative action by or in the right of such corporation) by reason of the fact that such person is or was a director, officer, employee or agent of such corporation, or serving at the request of such corporation in such capacity for another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding, if such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of such corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful.
The DGCL also permits indemnification by a corporation under similar circumstances for expenses (including attorneys’ fees) actually and reasonably incurred by such persons in connection with the defense or settlement of a derivative action or suit, except that no indemnification shall be made in respect of any claim, issue or matter as to which such person shall have been adjudged to be liable to such corporation unless the Delaware Court of Chancery or the court in which such action or suit was brought shall determine upon application that such person is fairly and reasonably entitled to indemnity for such expenses which such court shall deem proper.
To the extent a present or former director or officer is successful in the defense of such an action, suit or proceeding referenced above, or in defense of any claim, issue or matter therein, a corporation is required by the DGCL to indemnify such person for actual and reasonable expenses incurred in connection therewith. Expenses (including attorneys’ fees) incurred by such persons in defending any action, suit or proceeding may be paid in advance of the final disposition of such action, suit or proceeding upon in the case of a current officer or director, receipt of an undertaking by or on behalf of such person to repay such amount if it is ultimately determined that such person is not entitled to be so indemnified.
The DGCL provides that the indemnification described above shall not be deemed exclusive of other indemnification that may be granted by a corporation pursuant to its bylaws, disinterested directors’ vote, stockholders’ vote and agreement or otherwise.
Section 102(b)(7) of the DGCL enables a corporation, in its certificate of incorporation or an amendment thereto, to eliminate or limit the personal liability of a director to the corporation or its stockholders for monetary damages for violations of the directors’ fiduciary duty, except (i) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) pursuant to Section 174 of the DGCL (providing for liability of directors for unlawful payment of dividends or unlawful stock purchases or redemptions) or (iv) for any transaction from which a director derived an improper personal benefit. The Registrant’s certificate of incorporation provides for such limitations on liability for its directors.
The DGCL also provides corporations with the power to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of such corporation, or is or was serving at the request of such corporation in a similar capacity for another corporation, partnership, joint venture, trust or other enterprise, against any liability asserted against him or her in any such capacity or arising out of his or her status as such, whether or not the corporation would have the power to indemnify him or her against such liability as described above.
The Registrant’s amended and restated certificate of incorporation requires the Registrant to indemnify and hold harmless, to the fullest extent permitted by applicable law as it presently exists or may hereafter be amended, any person (a “covered person”) who was or is made or is threatened to be made a party or is otherwise involved in any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (a “proceeding”) by reason of the fact that he or she is or was a director, officer or member of a committee of the Registrant, or, while a director or officer of the Registrant, is or was serving at the request of the Registrant as a director or officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise or non-profit entity, including service with respect to employee benefit plans, against all liability and loss suffered and expenses (including attorneys’ fees), judgment, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with a proceeding.
In addition, under the Registrant’s amended and restated certificate of incorporation, in certain circumstances, the Registrant shall pay the expenses (including attorneys’ fees) incurred by a covered person in defending a proceeding in advance of the final disposition of such proceeding; provided, however, that the Registrant shall not be required to advance any expenses to a person against whom the Registrant directly brings an action, suit or proceeding alleging that such person (1) committed an act or omission not in good faith or (2) committed an act of intentional misconduct or a knowing violation of law. Additionally, an advancement of expenses incurred by a covered person shall be made only upon delivery to the Registrant of an undertaking, by or on behalf of such covered person, to repay all amounts so advanced if it shall ultimately be determined by final judicial decision from which there is no further right to appeal or otherwise in accordance with Delaware law that such covered person is not entitled to be indemnified for such expenses.
In addition, the Registrant has entered into indemnification agreements with its directors and executive officers that provide for additional indemnification protections, which form of agreement has been filed as an exhibit to this registration statement.
Item 15. Recent Sales of Unregistered Securities.
Except as set forth below, in the three years preceding the filing of this Registration Statement, the Registrant has not issued any securities that were not registered under the Securities Act:
In December 2021, the Company entered into a Simple Agreement for Future Equity (“SAFE”) and received total proceeds of $400,000. The amount invested was convertible into equity instruments upon the closing of a subsequent equity financing. The SAFE investor also received a warrant to purchase 323,000 shares of common stock with a 7-year exercise period and an exercise price $0.20 per share.
On March 21, 2022, the Company entered into the Exchange Agreement with the Company’s convertible note holders, Series A preferred stock shareholders, option holders and common stockholders at the time, to convert all outstanding investments into the Company’s common stock for a fixed number of shares. Upon execution of the Exchange Agreement, there were 246,814 shares of common stock and 3,197 warrants to purchase common stock at an exercise price of $0.21 per share issued to existing equity and debt holders. A total of 52,883 shares of common stock were forfeited upon the execution of the Exchange Agreement.
In January and February of 2022, the Company issued a total of 292,000 shares of common stock to the Board of Directors, Officers and Scientific Advisor, as compensation.
In March 2022, the Company completed a common stock offering, in which the Company sold 50,000 shares of common stock for gross proceeds of $2,000,000 and issued 2,300 warrants to purchase common stock at an exercise price of $40.00 per share. In addition, 1,150 shares of common stock were issued as broker compensation.
In March 2023, the Company completed a common stock offering in which the Company sold 16,875 shares of common stock for net proceeds of $675,000.
From April to June 2023, the Company completed a common stock offering, in which the Company sold 71,001 shares of common stock for gross proceeds of $2,840,000.
During the year ended March 31, 2024 and prior to the Company’s initial public offering, the Company issued the following unregistered securities: i) $2.0 million in unsecured, non-interest bearing convertible promissory notes (the Notes) and accompanying warrants (the Bridge Financing Warrants). The Notes provided that, on the closing date of the IPO, the outstanding principal would be automatically converted into common stock at the conversion price of $40.00. Each dollar in principal amount of Notes purchased were accompanied by a five-year Bridge Financing Warrant to purchase approximately 0.0125 shares of common stock with an exercise price of $20.00 per share; and ii) 1,750 shares of restricted stock for a consultant for purposes of providing business advisory services.
On July 7, 2023, the Company entered into a Termination Agreement with the Licensee in exchange for the issuance, upon the closing of the Company’s IPO within one year of the agreement’s execution, of a warrant to purchase shares of the Company for a variable number of shares based on a value of $8.0 million. Upon the closing of the Company’s IPO on January 29, 2024, the Company issued the licensee a warrant to purchase 80,000 shares, which were issued at an assumed valuation of $100.00 per share for a fixed value of $8.0 million. The warrants are exercisable at a price of $0.02 per share and may be exercised any time after the issuance date, subject to a beneficial ownership limitation, and expire five years from the original issuance.
On September 1, 2023, the Company issued a warrant to purchase 1,000 shares of common stock to a consultant providing investor relation advisory services.
On June 17, 2024, the Company entered into an employment agreement with Brad Hauser. Pursuant to the agreement, Mr. Hauser was granted a ten-year option (the “Inducement Options”) to purchase 45,000 shares of common stock at an exercise price equal to the closing price of the Company’s common stock on the date of the employment agreement. The Inducement Options were granted outside of the Company’s 2023 Stock Plan as an inducement material to Mr. Hauser’s entering into employment with the Company in accordance with Nasdaq Stock Market Listing Rule 5635(c)(4).
On July 10, 2024, as consideration for a license agreement, the Company agreed to issue 12,500 shares of its common stock.
All of the securities above were issued in reliance on the exemption provided by Section 4(a)(2) of the Securities Act for the offer and sale of securities not involving a public offering, and/or Regulation D promulgated under the Securities Act.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits:
|
Exhibit |
Description of Document |
|
|
1.1* |
||
|
3.1 |
||
|
3.2 |
||
|
4.1 |
||
|
4.2 |
||
|
4.3* |
||
| 4.4* | Form of Series A Warrant | |
| 4.5* | Form of Representative Warrant | |
| 4.6* | Warrant Agency Agreement | |
|
5.1* |
||
|
10.1** |
||
|
10.2** |
||
|
10.3** |
||
|
10.4** |
||
|
10.5 |
||
|
10.6 |
||
|
10.7 |
||
|
10.8+ |
||
|
10.9 |
||
|
10.10 |
||
|
10.11** |
||
|
10.12** |
||
|
10.13 |
||
|
23.1* |
||
|
23.2* |
||
|
24.1* |
||
|
107* |
||
|
* |
Filed herewith. |
|
** |
Management contract or compensatory plan, contract or arrangement. |
|
+ |
Pursuant to Item 601(b)(10)(iv) of Regulation S-K promulgated by the SEC, certain portions of this exhibit have been redacted. The Company hereby agrees to furnish supplementally to the SEC, upon its request, an unredacted copy of this exhibit. |
(b) Consolidated Financial Statement Schedules: All schedules are omitted because the required information is inapplicable or the information is presented in the consolidated financial statements and the related notes.
Item 17. Undertakings
(a) The undersigned Registrant hereby undertakes that:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement;
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
provided, however, that paragraphs (i), (ii) and (iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to Section 13 or Section 15(d) of the Exchange Act that are incorporated by reference in this Registration Statement or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the Registration Statement.
(2) That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§230.424 of this chapter);
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b) The undersigned Registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Exchange Act (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Exchange Act) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(c) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer, or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(d) The undersigned Registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
SIGNATURES
Pursuant to the requirements of the Securities Act, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Houston, Texas, on November 1, 2024.
|
|
AUTONOMIX MEDICAL, INC. (Registrant) |
|
|
|
|
|
|
|
|
|
By: |
/s/ Brad Hauser |
|
|
|
|
Brad Hauser |
|
|
|
|
Chief Executive Officer and President |
|
KNOW ALL MEN AND WOMEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints either Brad Hauser or Trent Smith, her or his true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution for her or him and in her or his name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this Registration Statement, and any subsequent registration statements pursuant to Rule 462 of the Securities Act of 1933 and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that each of said attorney-in-fact or her or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed below by the following persons in the capacities and on the dates indicated:
|
Date: November 1, 2024 |
By: |
/s/ BRAD HAUSER |
|
Brad Hauser |
||
|
Date: November 1, 2024 |
/s/ TRENT SMITH |
|
|
Trent Smith Chief Financial Officer (Principal Financial and Accounting Officer) |
||
|
Date: November 1, 2024 |
/s/ WALTER KLEMP |
|
|
Walter Klemp Executive Chairman of the Board of Directors |
||
|
Date: November 1, 2024 |
/s/ LORI BISSON |
|
|
Lori Bisson |
||
|
Date: November 1, 2024 |
/s/ JONATHAN FOSTER |
|
|
Jonathan Foster Director |
||
|
Date: November 1, 2024 |
/s/ DAVID ROBINS |
|
|
David Robins |
||
|
Date: November 1, 2024 |
/s/ CHRISTOPHER CAPELLI, MD |
|
|
Christopher Capelli |
