
全球牙科領袖 2024年10月 附件 99.2

前瞻性聲明和非依據通用會計原則(GAAP)計算的財務指標安全港聲明根據1995年《私人證券訴訟改革法》 本介紹包含根據聯邦證券法律的前瞻性聲明,包括但不限於任何關於我們的期望、計劃、意圖、策略或前景的聲明。我們通常使用“可能”、“將”、“期望”、“相信”、“預期”、“計劃”、“估算”、“項目化”、“假定”、“指導”、“目標”、“預测”、“視為”、“尋求”、“應該”、“可能”、“將會”、“預測”、“潛力”、“策略”、“未來”、“機會”、“努力”、“致力於”、“繼續”、“追踪”、“期待”、“樂觀”等表達來識別前瞻性聲明。除了歷史或當前事實的陳述外,所有陳述均被視為前瞻性聲明。此類陳述是基於管理層當前的信念、期望和假設,並受到可能導致實際結果和結果與前瞻性聲明顯著不同的重大風險、不確定性和情況變化的影響。這些風險、不確定性和情況變化包括但不限於: 依賴新產品開發、技術進步和創新;產品及服務的產品類別或區域銷售組合的轉變;原材料及產品的供應和價格;來自競爭對手、客戶、牙科診所和保險提供者的價格壓力;由於人口結構變化或其他因素導致的客戶對我們產品及服務的需求變化等因素導致的改變;與影響我們在美國和國際業務上的政府法律法規的變化及遵循相關的挑戰,包括美國食品和藥物管理局和外國政府監管機構的規定,如對產品審批有更嚴格要求的規定;競爭;醫改措施的影響;第三方支付方降低報銷水平;由政府機構、立法機構、私營部門和醫護團體購買組織贊助的成本管控措施,包括中國的基於成交量的採購過程;成本和開支的控制;依賴少數供應商提供關鍵原材料和外包活動的能力;取得和保持足夠的知識產權保護的能力;我們訊息技術系統或產品的侵害或失敗,包括受到網絡攻擊、未經授權訪問或盜竊的情況;保留營銷我們產品的獨立代理商和經銷商的能力;吸引、留住和培養我們業務所需的高技能員工的能力;企業併購對與客戶、供應商和貸款人的關係及對我們的營運結果和業務的一般影響;形成和實施聯盟的能力;由稅收改革措施產生的稅務義務變化,包括歐盟對國家援助的規則,或納稅機構的審查;產品責任、知識產權和商業訴訟損失;一般行業和市場條件的變化,包括國內和國際增長率的變化;一般國內和國際經濟狀況的變化,包括通貨膨脹、利率和匯率波動;全球大流行等對全球經濟、我們的業務及運營以及我們的供應商和客戶的業務和運營產生的不利公共衛生事件的影響,包括選擇性程序的推遲和我們收取應收款項的能力;及歐元區國家持續的財政和政治不確定性對受影響國家的應收款項收取能力的影響等。提醒您不要依賴這些前瞻性陳述,因為無法保證這些前瞻性陳述將被證明準確。前瞻性陳述僅於其發表日期有效,我們明確否認有任何意圖或義務更新或修訂任何前瞻性陳述,無論是因為新信息、未來事件或其他原因。 非依據通用會計原則(GAAP)計算的財務指標本介紹包含未按照美國通用會計原則(GAAP)計算的財務指標,因為它們是我們的管理層評估我們業績的依據。盡管我們認為出於同樣的原因這些指標可能對投資者有用,但這些財務指標不應被視為我們的財務狀況、業績或流動性的代替品。此外,這些財務指標可能不可與其他公司使用的類似指標相比。在本介紹的附錄中,我們提供了對這些非依據GAAP指標的進一步描述以及這些指標與最直接可比的GAAP指標的調解。
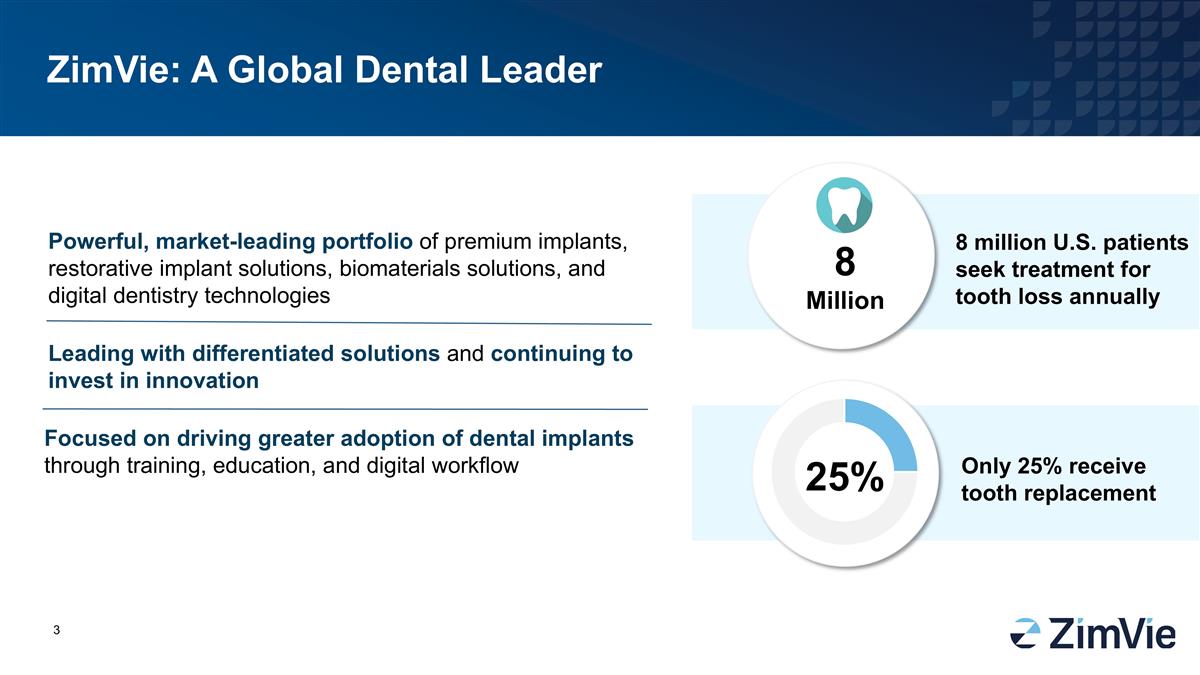
ZimVie: 一個全球貨幣牙科領導者 強大的、市場領先的高端植牙、修復植牙解決方案、生物材料解決方案和數碼牙科技術 800萬 800萬 美國患者每年尋求牙齒缺失治療 25% 僅有25% 接受牙齒 補習 通過培訓、教育和數碼工作流來推動牙科植入物的更廣泛應用,引領獨特解決方案,並持續投資於創新
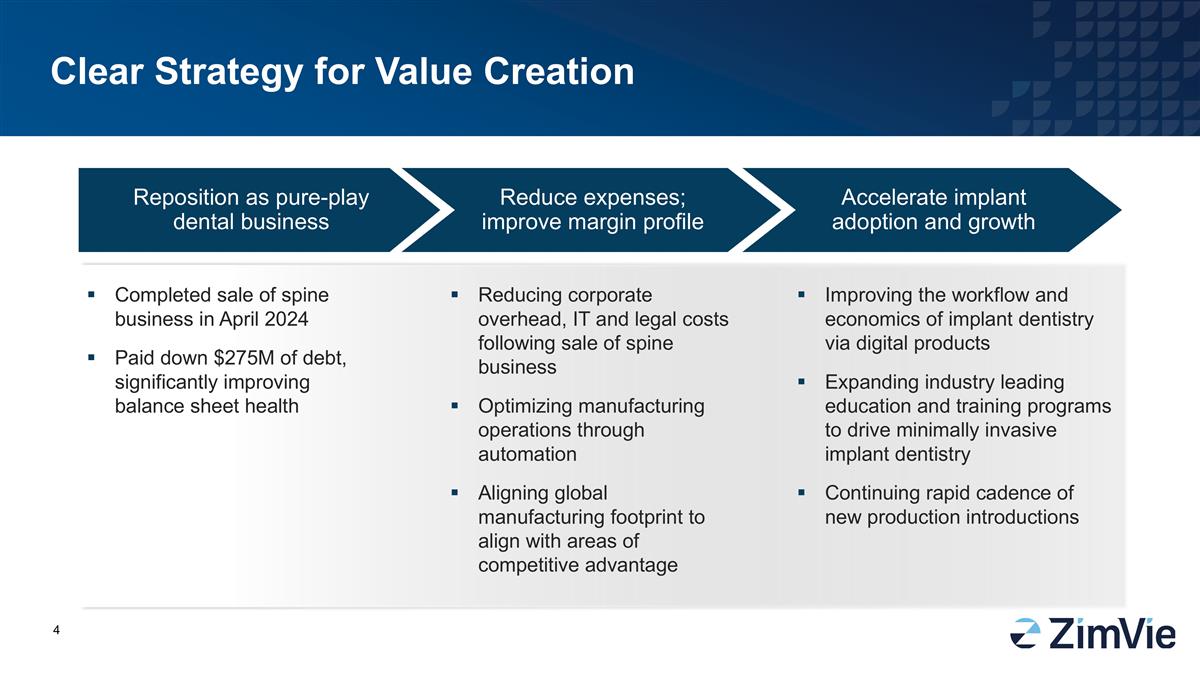
清晰的創造價值的策略 2024年4月完成了脊柱業務的出售,償還了27500萬美元的債務,顯著改善了資產負債表健康狀況,跟著脊柱業務出售後減少企業頭部、資訊科技和法律成本,透過自動化優化製造業務合作對齊全球製造版圖,以與競爭優勢相同的領域進行調整,透過數位產品改善植牙醫學的工作流程和經濟學,擴大行業領先的教育培訓計畫以推動微創植牙醫學,持續快節奏推出新產品,重定位成為純粹的牙科業務,降低支出、改善利潤概況,加速植牙的接受和成長。
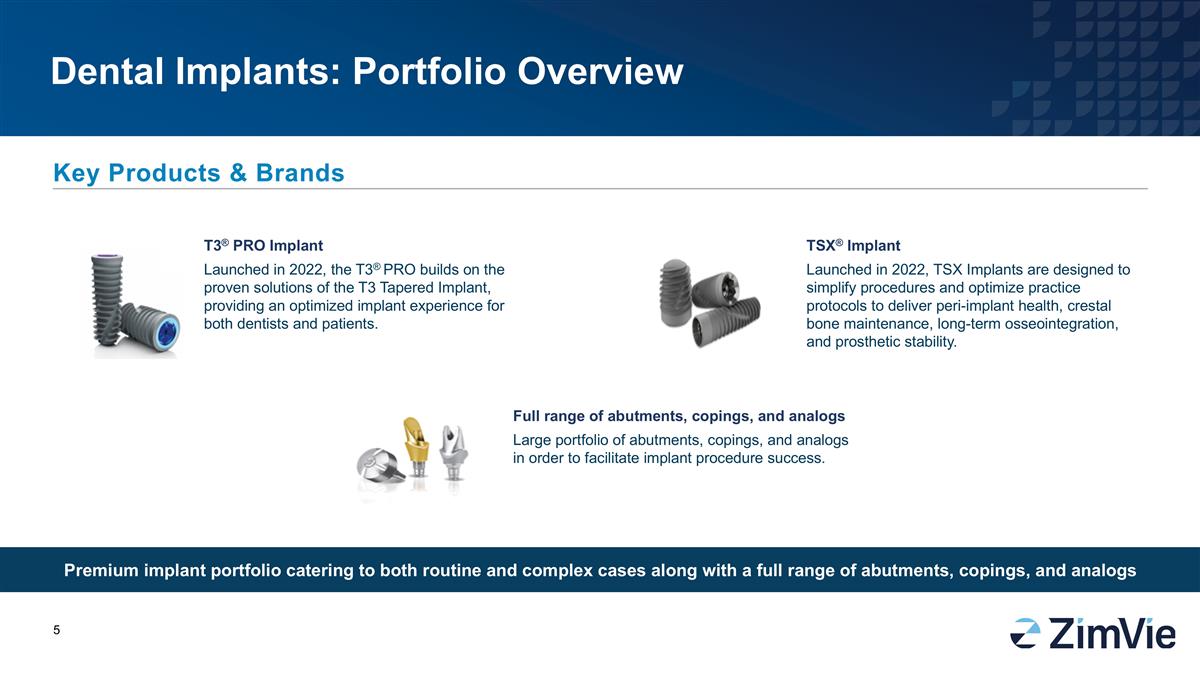
牙科植牙:產品組合概述 提供適用於常規和複雜案例的高級植牙產品組合,包括全系列後牙柱、帽層和模型 主要產品和品牌 tsx®植牙 2022年推出的tsx植牙旨在簡化程序並優化實踐協議,以提供牙周植牙健康、牙齦骨保持、長期骨融合和修復穩定性。 T3® PRO植牙 2022年推出的T3® PRO建立在T3錐形植牙的成熟解決方案基礎上,為牙醫和患者提供優化的植牙體驗。全系列後牙柱、帽層和模型 大量的後牙柱、帽層和模型,以幫助順利進行植牙程序。

Biomaterials: Portfolio Overview Biomaterial solutions that are used for soft tissue and bone rehabilitation, helping build sufficient bone necessary for dental implant surgery Key Products & Brands Barrier Membranes By providing a reliable barrier during the critical phases of wound healing, these membranes help maintain bone growth material. Puros® Allograft Products Products used in implant procedures to provide a foundation for the implant and create a desirable aesthetic outcome. Puros® Allograft Bone Block Human-donor sourced bone graft material that allows patients with damaged or inadequate bone quality to be provided with a stable surface for implant application. Xenograft and Synthetic Bone Grafts Synthetic bone material that can be used to create a suitable surface of implantation.
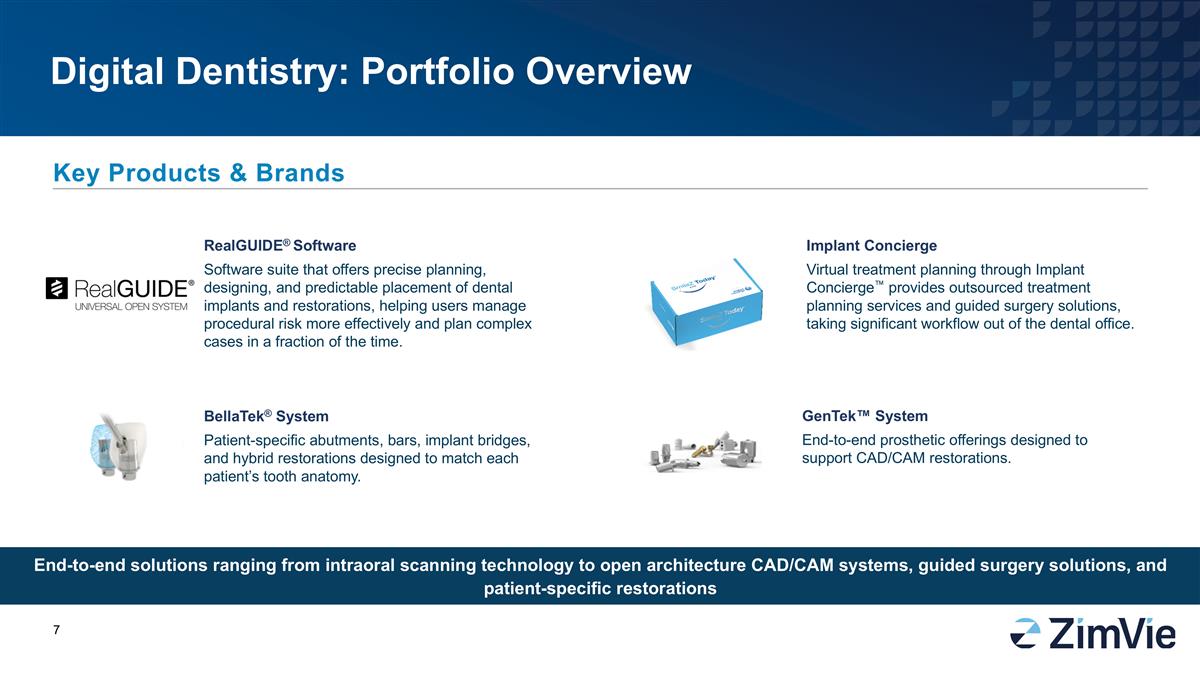
Digital Dentistry: Portfolio Overview End-to-end solutions ranging from intraoral scanning technology to open architecture CAD/CAM systems, guided surgery solutions, and patient-specific restorations Key Products & Brands GenTek™ System End-to-end prosthetic offerings designed to support CAD/CAM restorations. Implant Concierge Virtual treatment planning through Implant Concierge™ provides outsourced treatment planning services and guided surgery solutions, taking significant workflow out of the dental office. RealGUIDE® Software Software suite that offers precise planning, designing, and predictable placement of dental implants and restorations, helping users manage procedural risk more effectively and plan complex cases in a fraction of the time. BellaTek® System Patient-specific abutments, bars, implant bridges, and hybrid restorations designed to match each patient’s tooth anatomy.

Revitalizing the Portfolio with Recent Launches T3® PRO Implant Encode® Emergence Healing Abutment TSX® Implant Azure™ Multi-Platform Solutions Portfolio RegenerOss® Cortico–Cancellous Particulate RegenerOss® Bone Graft Plug Biotivity™ A/C Plus Membrane RealGUIDE® 5.4 Software CAD/CAM Workflow Systems MEDIT Intraoral Scanners Dental Implants Biomaterials Digital Dentistry Biotivity™ Hyaluronic Acid

Virtual Treatment Planning Custom Surgical Guide Kits Delivering digital workflow enhancements to save clinician time and improve patient satisfaction AI facilitated reconstruction procedures require 3 fewer hours of human labor* ZimVie Encode Emergence workflow reduces chair time and saves one restorative impression appointment Seeing rapid adoption of guided surgery software End-to-End Solutions Save Time and Improve the Clinician and Patient Experience *Internal data
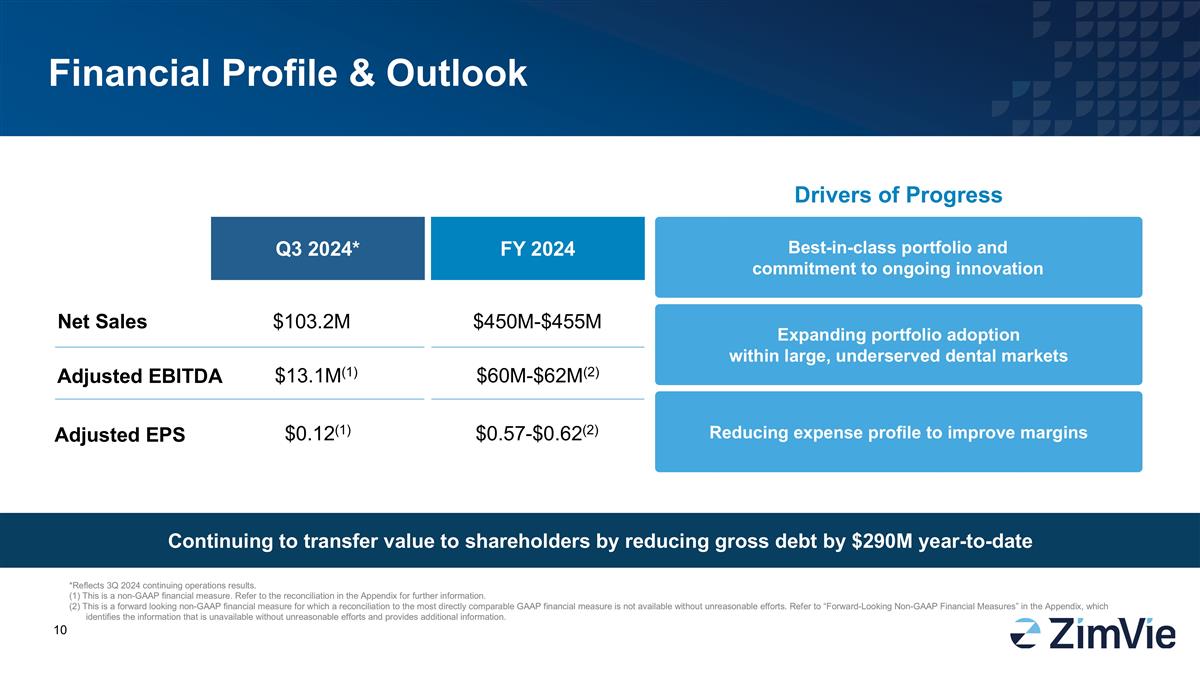
Financial Profile & Outlook Net Sales Adjusted EBITDA Q3 2024* FY 2024 $103.2M $13.1M(1) $450M-$455M $60M-$62M(2) Drivers of Progress Expanding portfolio adoption within large, underserved dental markets Reducing expense profile to improve margins Best-in-class portfolio and commitment to ongoing innovation Adjusted EPS $0.12(1) $0.57-$0.62(2) Continuing to transfer value to shareholders by reducing gross debt by $290M year-to-date *Reflects 3Q 2024 continuing operations results. (1) This is a non-GAAP financial measure. Refer to the reconciliation in the Appendix for further information. (2) This is a forward looking non-GAAP financial measure for which a reconciliation to the most directly comparable GAAP financial measure is not available without unreasonable efforts. Refer to “Forward-Looking Non-GAAP Financial Measures” in the Appendix, which identifies the information that is unavailable without unreasonable efforts and provides additional information.

Committed to Executing Strategic Transformation Commercialize new product introductions across major geographies Drive digital workflow adoption to expand implant adoption Address and reduce stranded costs Optimize manufacturing & supply chain capabilities Position the business for sustainable growth Innovate digital workflow to drive efficiency and outcomes for the most complex implant procedures Expand geographically with direct representation Continue to invest in training and education to drive adoption Transformed to pure-play dental business Paid down $275M of debt Launched RealGUIDE® software update Introduced GenTek Restorative Components (U.S.) Recent Accomplishments Current Priorities Market Expansion Opportunities

Appendix

Note on Non-GAAP Financial Measures This presentation includes non-GAAP financial measures that differ from financial measures calculated in accordance with U.S. generally accepted accounting principles (“GAAP”). These non-GAAP financial measures may not be comparable to similar measures reported by other companies and should be considered in addition to, and not as a substitute for, or superior to, other measures prepared in accordance with GAAP. Adjusted EBITDA is a non-GAAP financial measure provided in this presentation for certain periods and is calculated by excluding certain items from net loss from Continuing Operations on a GAAP basis, as detailed in the reconciliations presented later in this presentation. Adjusted EBITDA margin is Adjusted EBITDA divided by third party net sales from Continuing Operations for the applicable period. Adjusted diluted earnings (loss) per share is a non-GAAP financial measure provided in this presentation for certain periods and is calculated by excluding the effects of certain items from diluted earnings (loss) per share on a GAAP basis, as detailed in the reconciliations presented later in this presentation. Reconciliations of these non-GAAP measures to the most directly comparable GAAP financial measures are included in this presentation. Management uses non-GAAP financial measures internally to evaluate the performance of the business. Additionally, management believes these non-GAAP measures provide meaningful incremental information to investors to consider when evaluating the performance of the company. Management believes these measures offer the ability to make period-to-period comparisons that are not impacted by certain items that can cause dramatic changes in reported income but that do not impact the fundamentals of our operations. The non-GAAP measures enable the evaluation of operating results and trend analysis by allowing a reader to better identify operating trends that may otherwise be masked or distorted by these types of items that are excluded from the non-GAAP measures. Forward-Looking Non-GAAP Financial Measures This presentation also includes certain forward-looking non-GAAP financial measures for the year ending December 31, 2024. We calculate forward-looking non-GAAP financial measures based on internal forecasts that omit certain amounts that would be included in GAAP financial measures. We have not provided quantitative reconciliations of these forward-looking non-GAAP financial measures to the most directly comparable forward-looking GAAP financial measures because the excluded items are not available on a prospective basis without unreasonable efforts. For example, the timing of certain transactions is difficult to predict because management’s plans may change. In addition, the company believes such reconciliations would imply a degree of precision and certainty that could be confusing to investors. It is probable that these forward-looking non-GAAP financial measures may be materially different from the corresponding GAAP financial measures.
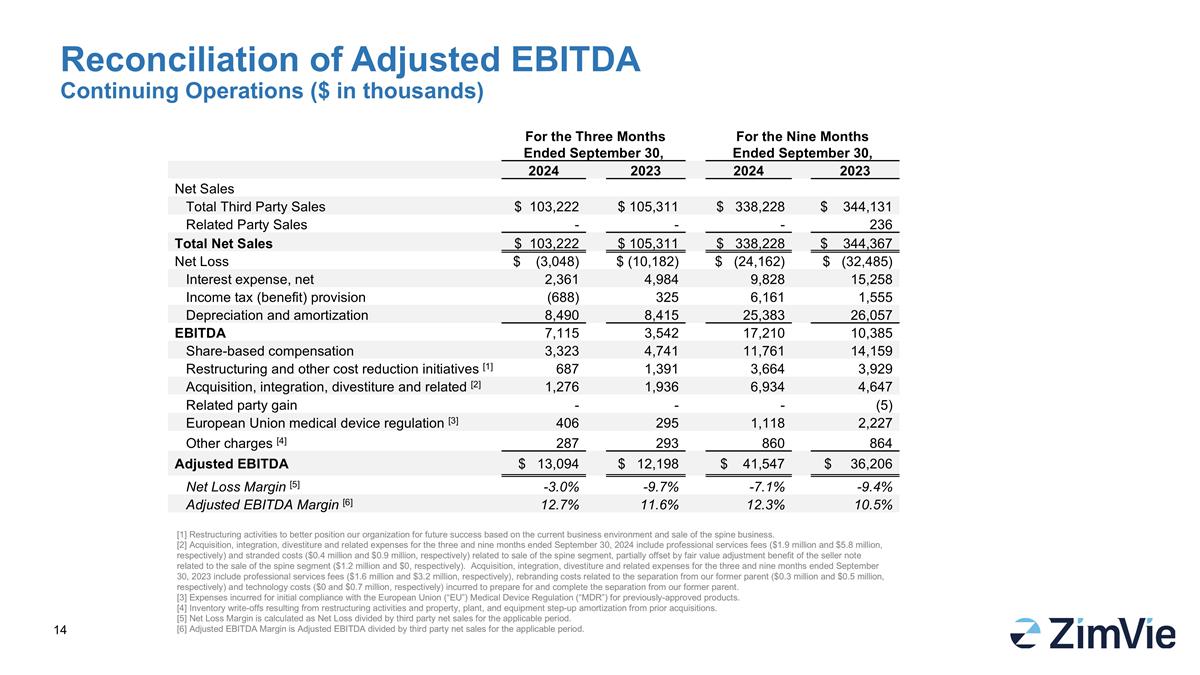
Reconciliation of Adjusted EBITDA Continuing Operations ($ in thousands) [1] Restructuring activities to better position our organization for future success based on the current business environment and sale of the spine business. [2] Acquisition, integration, divestiture and related expenses for the three and nine months ended September 30, 2024 include professional services fees ($1.9 million and $5.8 million, respectively) and stranded costs ($0.4 million and $0.9 million, respectively) related to sale of the spine segment, partially offset by fair value adjustment benefit of the seller note related to the sale of the spine segment ($1.2 million and $0, respectively). Acquisition, integration, divestiture and related expenses for the three and nine months ended September 30, 2023 include professional services fees ($1.6 million and $3.2 million, respectively), rebranding costs related to the separation from our former parent ($0.3 million and $0.5 million, respectively) and technology costs ($0 and $0.7 million, respectively) incurred to prepare for and complete the separation from our former parent. [3] Expenses incurred for initial compliance with the European Union (“EU”) Medical Device Regulation (“MDR”) for previously-approved products. [4] Inventory write-offs resulting from restructuring activities and property, plant, and equipment step-up amortization from prior acquisitions. [5] Net Loss Margin is calculated as Net Loss divided by third party net sales for the applicable period. [6] Adjusted EBITDA Margin is Adjusted EBITDA divided by third party net sales for the applicable period. For the Three Months Ended September 30, For the Nine Months Ended September 30, 2024 2023 2024 2023 Net Sales Total Third Party Sales $ 103,222 $ 105,311 $ 338,228 $ 344,131 Related Party Sales - - - 236 Total Net Sales $ 103,222 $ 105,311 $ 338,228 $ 344,367 Net Loss $ (3,048) $ (10,182) $ (24,162) $ (32,485) Interest expense, net 2,361 4,984 9,828 15,258 Income tax (benefit) provision (688) 325 6,161 1,555 Depreciation and amortization 8,490 8,415 25,383 26,057 EBITDA 7,115 3,542 17,210 10,385 Share-based compensation 3,323 4,741 11,761 14,159 Restructuring and other cost reduction initiatives [1] 687 1,391 3,664 3,929 Acquisition, integration, divestiture and related [2] 1,276 1,936 6,934 4,647 Related party gain - - - (5) European Union medical device regulation [3] 406 295 1,118 2,227 Other charges [4] 287 293 860 864 Adjusted EBITDA $ 13,094 $ 12,198 $ 41,547 $ 36,206 Net Loss Margin [5] -3.0% -9.7% -7.1% -9.4% Adjusted EBITDA Margin [6] 12.7% 11.6% 12.3% 10.5%
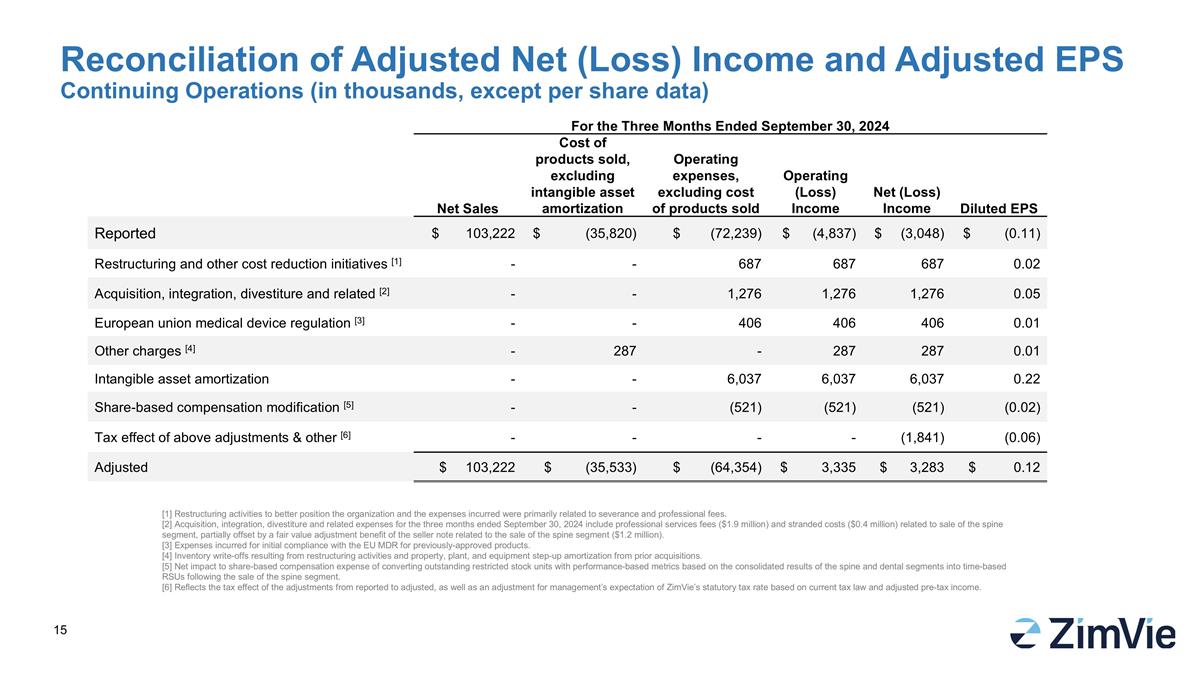
Reconciliation of Adjusted Net (Loss) Income and Adjusted EPS Continuing Operations (in thousands, except per share data) [1] Restructuring activities to better position the organization and the expenses incurred were primarily related to severance and professional fees. [2] Acquisition, integration, divestiture and related expenses for the three months ended September 30, 2024 include professional services fees ($1.9 million) and stranded costs ($0.4 million) related to sale of the spine segment, partially offset by a fair value adjustment benefit of the seller note related to the sale of the spine segment ($1.2 million). [3] Expenses incurred for initial compliance with the EU MDR for previously-approved products. [4] Inventory write-offs resulting from restructuring activities and property, plant, and equipment step-up amortization from prior acquisitions. [5] Net impact to share-based compensation expense of converting outstanding restricted stock units with performance-based metrics based on the consolidated results of the spine and dental segments into time-based RSUs following the sale of the spine segment. [6] Reflects the tax effect of the adjustments from reported to adjusted, as well as an adjustment for management’s expectation of ZimVie’s statutory tax rate based on current tax law and adjusted pre-tax income. For the Three Months Ended September 30, 2024 Net Sales Cost of products sold, excluding intangible asset amortization Operating expenses, excluding cost of products sold Operating (Loss) Income Net (Loss) Income Diluted EPS Reported $ 103,222 $ (35,820) $ (72,239) $ (4,837) $ (3,048) $ (0.11) Restructuring and other cost reduction initiatives [1] - - 687 687 687 0.02 Acquisition, integration, divestiture and related [2] - - 1,276 1,276 1,276 0.05 European union medical device regulation [3] - - 406 406 406 0.01 Other charges [4] - 287 - 287 287 0.01 Intangible asset amortization - - 6,037 6,037 6,037 0.22 Share-based compensation modification [5] - - (521) (521) (521) (0.02) Tax effect of above adjustments & other [6] - - - - (1,841) (0.06) Adjusted $ 103,222 $ (35,533) $ (64,354) $ 3,335 $ 3,283 $ 0.12