
2024年8月1日企业展示 附件99.2

前瞻性陈述和其他法律声明 本介绍包含有关Longboard Pharmaceuticals, Inc.(我们,Longboard或公司)的前瞻性陈述,包括关于:我们的愿景;商业机会和类似物;bexicaserin(LP352)和LP659的潜力,包括成为最佳类别,治疗适应症,具有差异化或下一代设计,选择性,特异性和其他特征,以及满足未满足的需求;预期的里程碑和时间表;DEEs的普遍存在性,与未满足需求相关的需求以及市场机会;bexicaserin第3阶段全球计划设计,启动时间和DEE前进道路;bexicaserin获得突破性疗法称号;LP659的广泛适用性,预测性数据,商业机会,下一代和选择性特征,作用机制和临床前数据,限制非靶效应的潜力,潜在的神经退行性疾病治疗领域,适应症和机会,顶线SAD数据和第1阶段MAD启动时间;我们的知识产权;我们获得监管批准和商业化我们的药物候选者的能力(以我们可能提出的方式或根本不提出);以及其他不是历史事实的声明,包括可能包含“将”,“可能”,“可以”,“愿”,“计划”,“预期”,“相信”,“潜力”,“目标”,“机会”以及类似词语的声明。对于此类陈述,我们声称受1995年《私人证券诉讼改革法》的保护。这些前瞻性陈述受到一系列风险,不确定性和假设的影响,包括但不限于:顶线或中期数据可能不反映特定研究或试验的完整或最终结果,并可能发生变化;非临床和临床数据繁多且详细,监管机构可能以不同于我们或其他人的方式解释或权衡数据的重要性,并得出与我们或其他人的不同结论,要求额外信息,在批准前或后之前有额外的建议或更改他们的指导意见或要求;我们运营历史有限,经历过净亏损的历史,预计在可预见的未来将继续发生净亏损,我们可能永远没有盈利;我们需要额外资本来资助我们的运营;临床和临床前药物开发涉及漫长和昂贵的过程,时间进程不确定且结果不明确;我们有多个产品候选药物,针对各种靶标适应症,我们可能会耗尽有限资源来追求特定产品候选者或适应症,并未能充分利用可能更具利润性的产品候选者或适应症;美国及其他领土的监管批准流程漫长,耗时且固有地不可预测,我们可能无法获得或维持监管批准以进行我们的临床试验(以我们提出的方式或根本不提出)或最终营销我们的产品候选者;获得突破性疗法称号不一定会导致更快的发展或监管审查或批准,并不意味着bexicaserin将获得Haosa与DEEs相关的癫痫或任何其他适应症的营销批准;涉及我们将商业化我们的产品候选者并在市场上竞争的风险;涉及我们的许可证和对他人的依赖的风险;涉及我们获得和维持知识产权保护及为我们的产品候选者自由运作的风险;涉及我们管理增长的能力的风险;以及其他在我们与美国证券交易委员会(SEC)的提交中披露的风险和因素。我们在一个竞争激烈且迅速变化的环境中运营。新的风险不断出现。我们的管理层无法预测所有风险,我们也无法评估所有因素对我们业务的影响,或任何因素的影响,或多个因素的结合可能导致实际结果与我们可能进行的任何前瞻性声明中包含的结果有实质区别。本介绍中讨论的前瞻性事件和情况可能不会发生,实际结果可能根据前瞻性声明中预期或暗示的情况发生实质且不利的差异。除法律要求外,我们对前瞻性陈述的准确性和完整性不承担责任,不对任何在本介绍之日后更新任何前瞻性陈述使这些陈述符合实际结果或我们期望的变化承担任何义务。本介绍中包含的或可能口头陪同的某些信息与基于第三方来源获得的研究,研究,出版物,调查和其他数据以及我们自己的内部估计和研究相关。虽然我们相信这些第三方研究,研究,出版物,调查和其他数据截至本介绍日可靠,但它们尚未经过独立验证,我们不对从第三方来源获得的任何信息的充分性,公平性,准确性或完整性作出任何陈述。本介绍讨论了尚未获得美国食品药品监督管理局(FDA)或其他任何监管机构批准的产品候选者bexicaserin和LP659。

我们的愿景是建立在20多年世界一流的GPCR研究的基础上。我们希望实现一个世界,在这个世界中毁灭性的神经系统疾病不再是毁灭性的。3 Longboard Pharmaceuticals 通过差异化创新的临床方法、相关的并购模拟 进行股权交易,在精神神经科和罕见疾病方面有着大胆而经验丰富的领导层,致力于CNS和罕见疾病,拥有具有差异化药代动力学/药效学和靶点结合的产品线,CNS项目具有巨大的商业机遇,其靶点得到广泛认可。
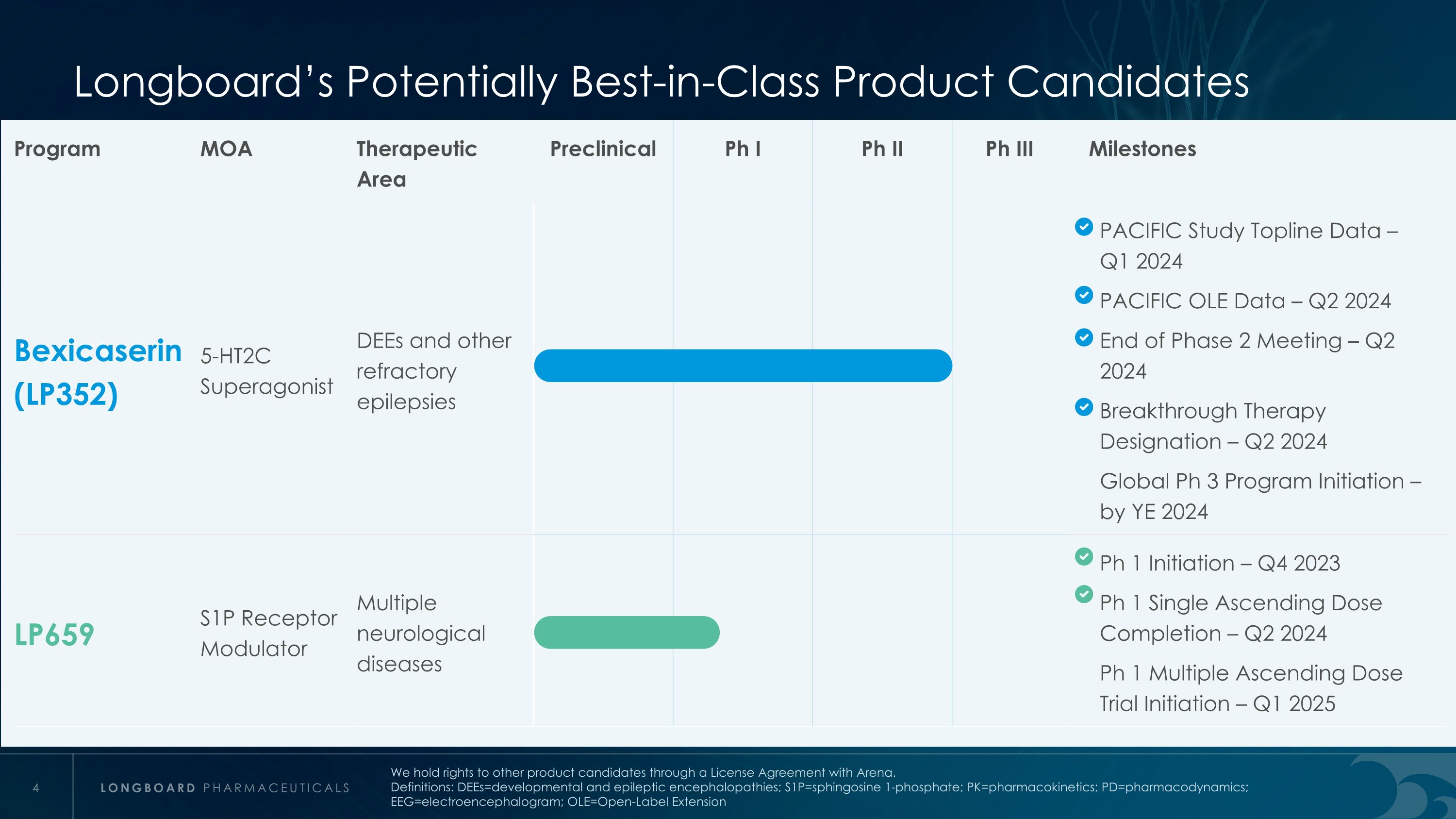
龙板潜在的最佳品类候选产品计划MOA治疗领域临床前阶段I期II期III期里程碑百哲卡塞琳(LP352)5-HT2C超激动剂DEEs和其他难治性癫痫PACIFIC研究头条数据 – 2024年第一季度PACIFIC OLE数据 – 2024年第二季度二期会议结束– 2024年第二季度突破性治疗指定 – 2024年第二季度全球三期项目启动 – 截止到2024年底LP659 S1P受体调节剂多种神经系统疾病I期启动– 2023年第四季度I期单升剂量完成– 2024年第二季度I期多升剂量试验启动 – 2025年第一季度我们通过与Arena签订的许可协议持有其他产品候选人的权利。定义:DEEs=发育性癫痫性和癫痫性脑病;S1P=鞘氨醇1磷酸酯;PK=药代动力学;PD=药效动力学;EEG=脑电图;OLE=开放标签延伸

发育性癫痫性脑病(DEE)概况
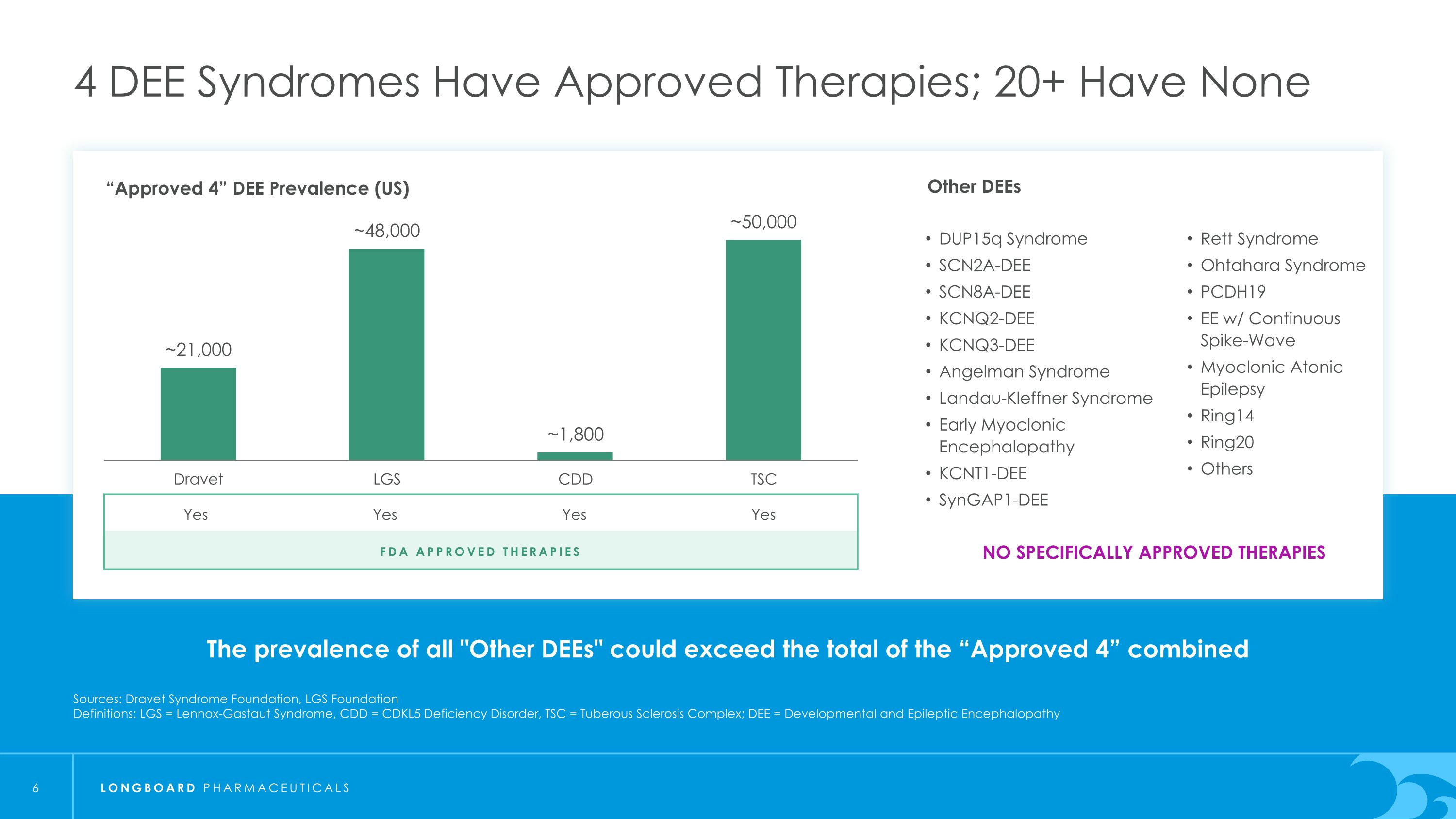
所有"其他DEE"的患病率可能超过“已批准的4种”合并的4种DEE综合征有已批准的疗法;20多种没有来源:Dravet综合征基金会,LGS基金会定义:LGS = Lennox-Gastaut综合征,CDD = CDKL5缺陷疾病,TSC = 结节性硬化症;DEE = 发育性和癫痫性脑病"已批准的4种"DEE患病率(美国)FDA批准的疗法是是是是不是没有特定批准的疗法其他DEEs DUP15q综合征SCN2A-DEESCN8A-DEEKCNQ2-DEEKCNQ3-DEEAngelman综合征Landau-Kleffner综合征早期肌阵挛性脑病KCNT1-DEESynGAP1-DEERett综合征Ohtahara综合征PCDH19带有持续性尖波-波群肌阵挛性弛缓癫痫Ring14Ring20其他
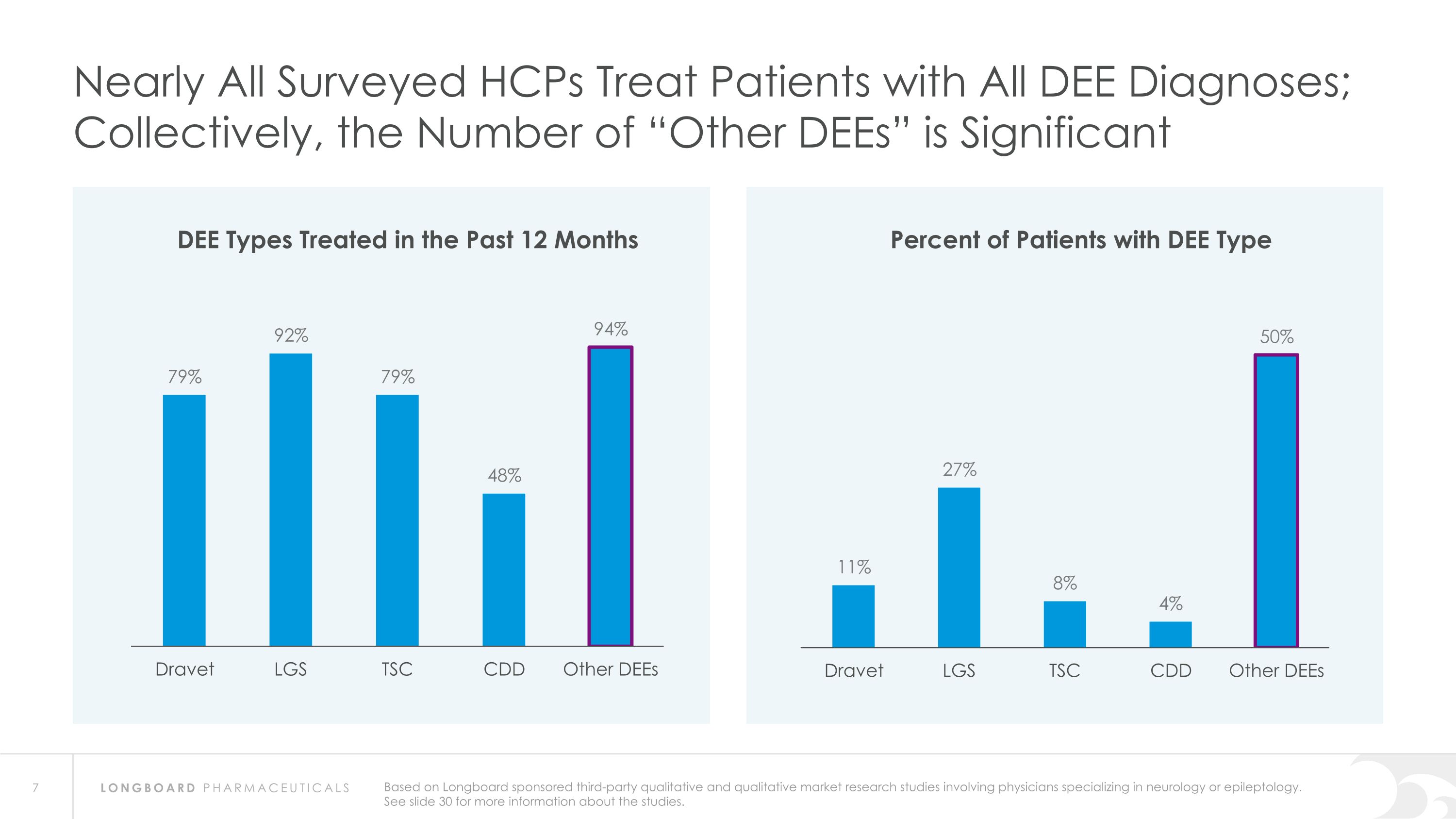
Nearly All Surveyed HCPs Treat Patients with All DEE Diagnoses; Collectively, the Number of “Other DEEs” is Significant DEE Types Treated in the Past 12 Months Percent of Patients with DEE Type Based on Longboard sponsored third-party qualitative and qualitative market research studies involving physicians specializing in neurology or epileptology. See slide 30 for more information about the studies.

Bexicaserin (LP352) Potential Best-in-Class 5-HT2C Superagonist - Entering a Ph 3 Program with the Goal of Treating a Broad Range of DEEs
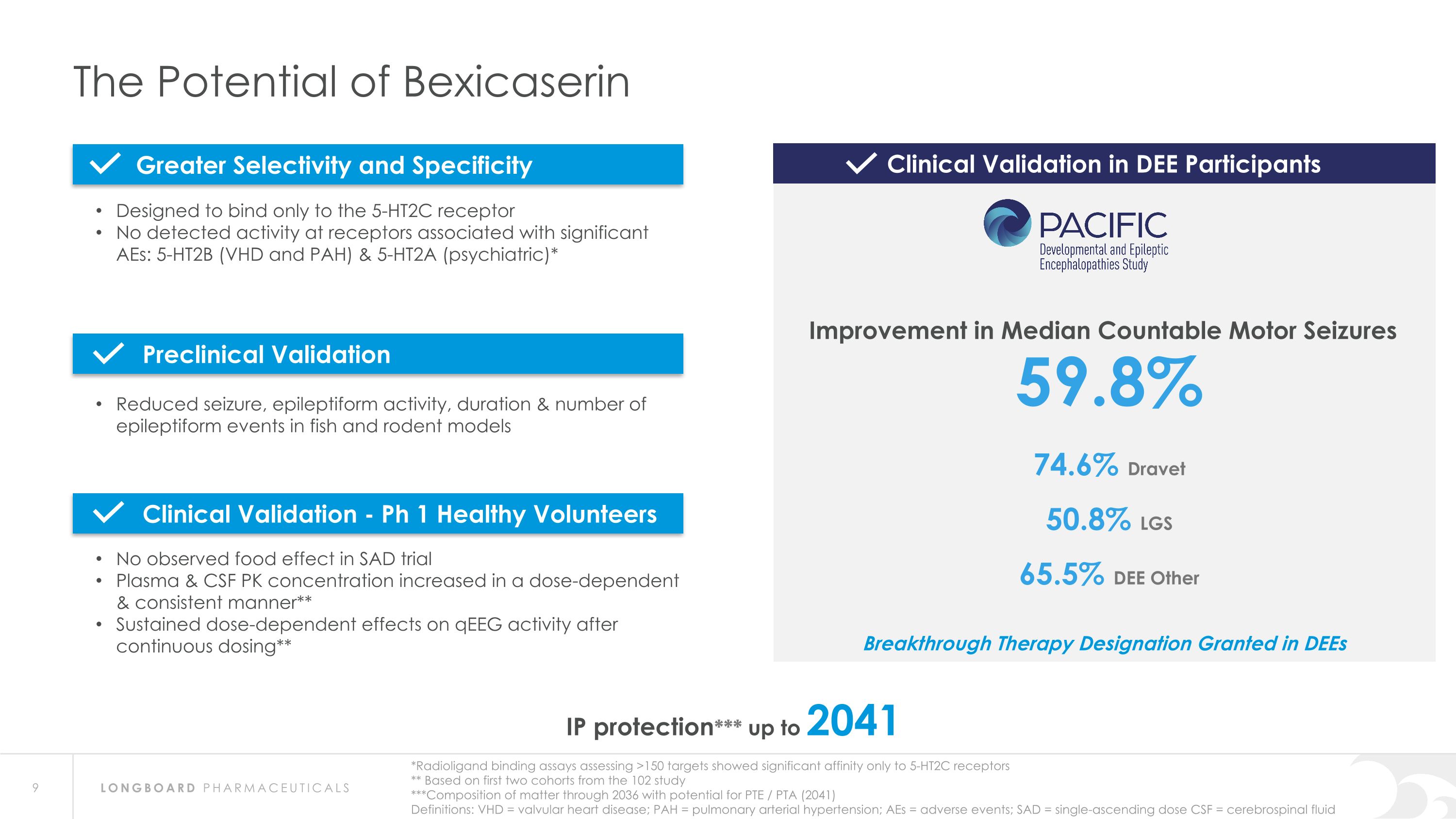
The Potential of Bexicaserin IP protection*** up to 2041 *Radioligand binding assays assessing >150 targets showed significant affinity only to 5-HT2C receptors ** Based on first two cohorts from the 102 study ***Composition of matter through 2036 with potential for PTE / PTA (2041) Definitions: VHD = valvular heart disease; PAH = pulmonary arterial hypertension; AEs = adverse events; SAD = single-ascending dose CSF = cerebrospinal fluid 59.8% 74.6% Dravet 50.8% LGS 65.5% DEE Other Designed to bind only to the 5-HT2C receptor No detected activity at receptors associated with significant AEs: 5-HT2B (VHD and PAH) & 5-HT2A (psychiatric)* Reduced seizure, epileptiform activity, duration & number of epileptiform events in fish and rodent models No observed food effect in SAD trial Plasma & CSF PK concentration increased in a dose-dependent & consistent manner** Sustained dose-dependent effects on qEEG activity after continuous dosing** Clinical Validation in DEE Participants Improvement in Median Countable Motor Seizures Greater Selectivity and Specificity Preclinical Validation Clinical Validation - Ph 1 Healthy Volunteers Breakthrough Therapy Designation Granted in DEEs
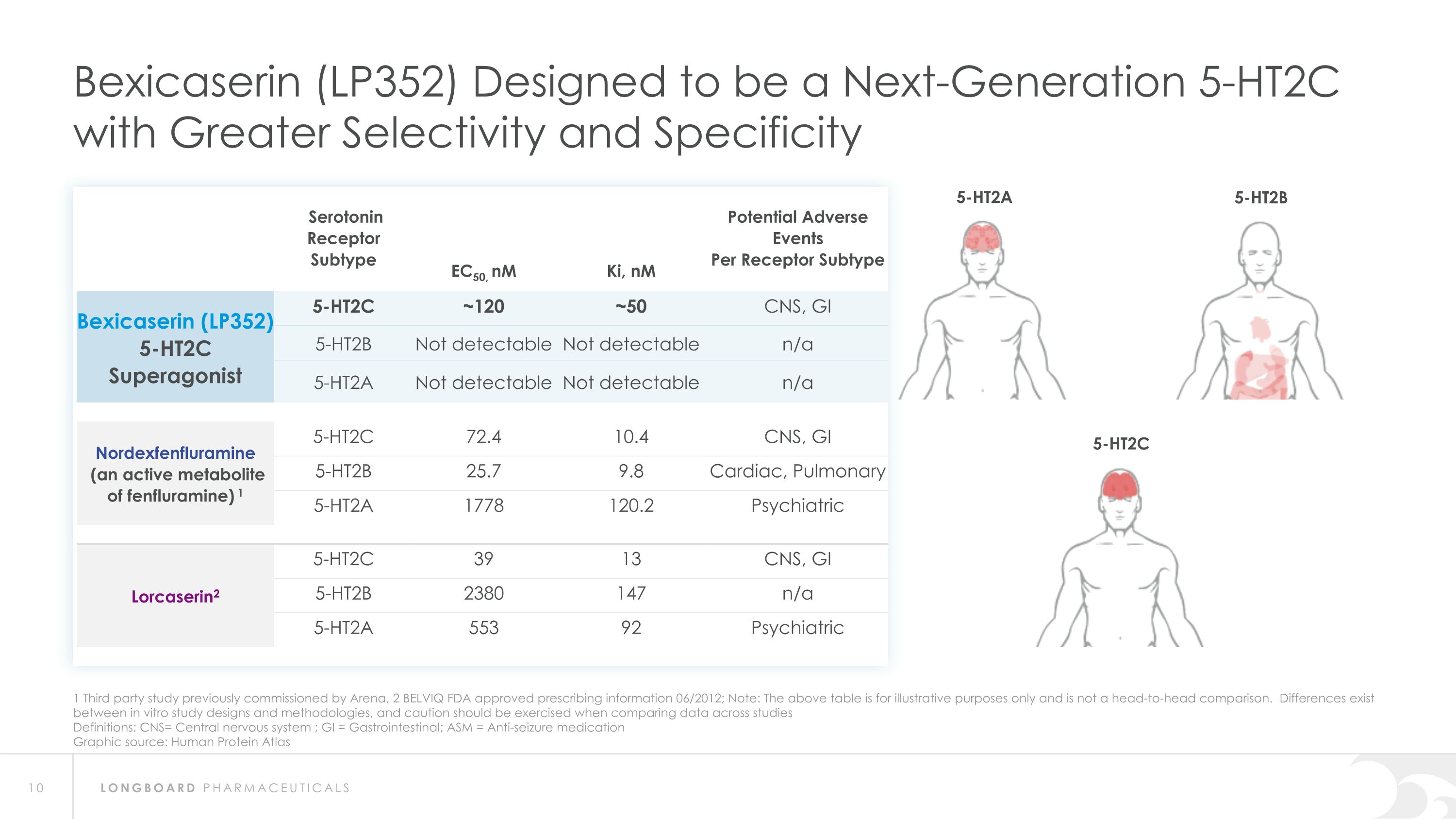
1 Third party study previously commissioned by Arena, 2 BELVIQ FDA approved prescribing information 06/2012; Note: The above table is for illustrative purposes only and is not a head-to-head comparison. Differences exist between in vitro study designs and methodologies, and caution should be exercised when comparing data across studies Definitions: CNS= Central nervous system ; GI = Gastrointestinal; ASM = Anti-seizure medication Graphic source: Human Protein Atlas Bexicaserin (LP352) Designed to be a Next-Generation 5-HT2C with Greater Selectivity and Specificity Serotonin Receptor Subtype EC50, nM Ki, nM Potential Adverse Events Per Receptor Subtype Bexicaserin (LP352) 5-HT2C Superagonist 5-HT2C ~120 ~50 CNS, GI 5-HT2B Not detectable Not detectable n/a 5-HT2A Not detectable Not detectable n/a Nordexfenfluramine (an active metabolite of fenfluramine) 1 5-HT2C 72.4 10.4 CNS, GI 5-HT2B 25.7 9.8 Cardiac, Pulmonary 5-HT2A 1778 120.2 Psychiatric Lorcaserin2 5-HT2C 39 13 CNS, GI 5-HT2B 2380 147 n/a 5-HT2A 553 92 Psychiatric 5-HT2A 5-HT2B 5-HT2C
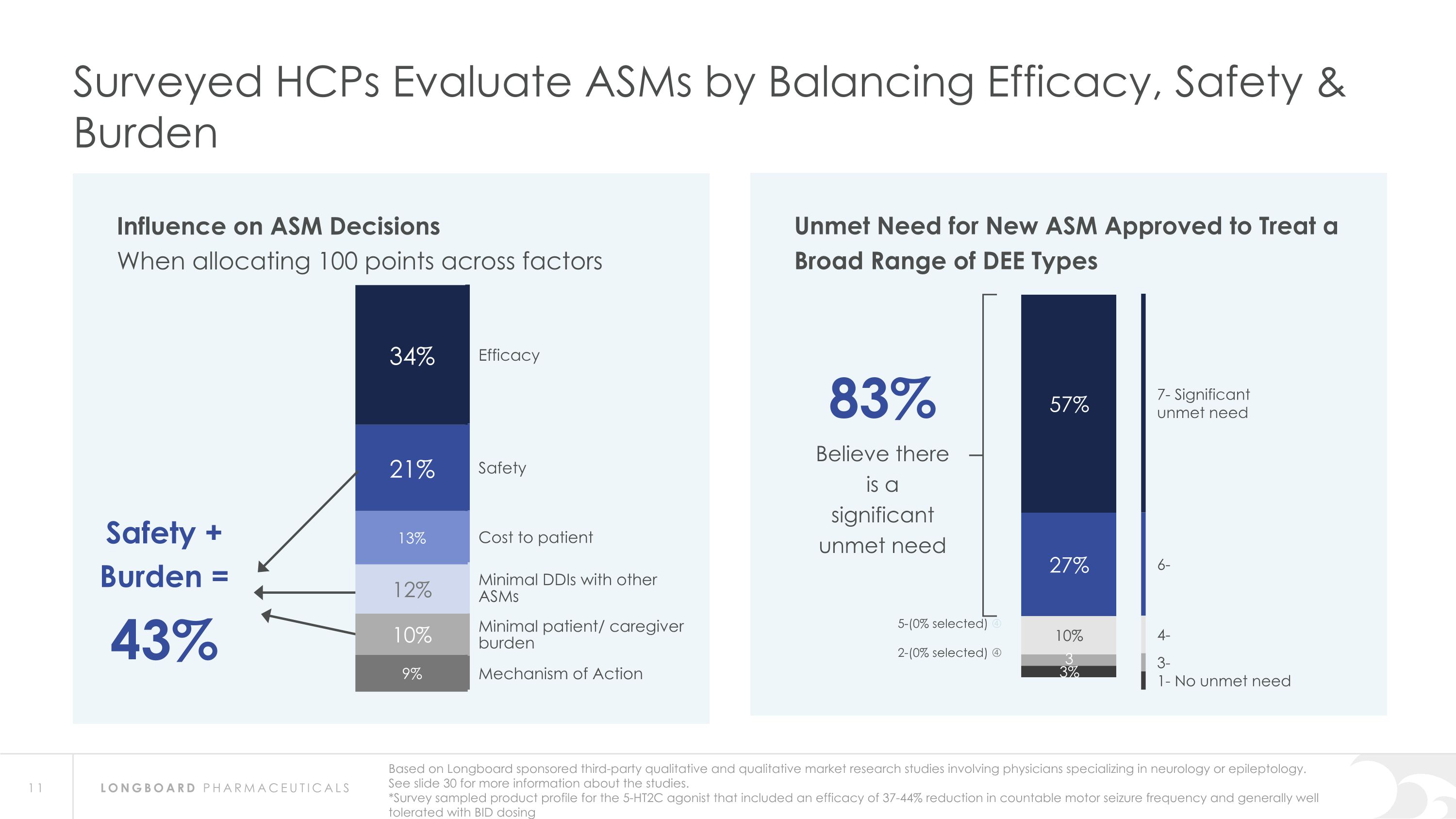
Influence on ASM Decisions When allocating 100 points across factors Surveyed HCPs Evaluate ASMs by Balancing Efficacy, Safety & Burden Efficacy Safety Cost to patient Minimal DDIs with other ASMs Minimal patient/ caregiver burden Mechanism of Action Safety + Burden = 43% Based on Longboard sponsored third-party qualitative and qualitative market research studies involving physicians specializing in neurology or epileptology. See slide 30 for more information about the studies. *Survey sampled product profile for the 5-HT2C agonist that included an efficacy of 37-44% reduction in countable motor seizure frequency and generally well tolerated with BID dosing Unmet Need for New ASM Approved to Treat a Broad Range of DEE Types 83% Believe there is a significant unmet need 7- Significant unmet need 6- 4- 3- 1- No unmet need 5-(0% selected) 2-(0% selected)
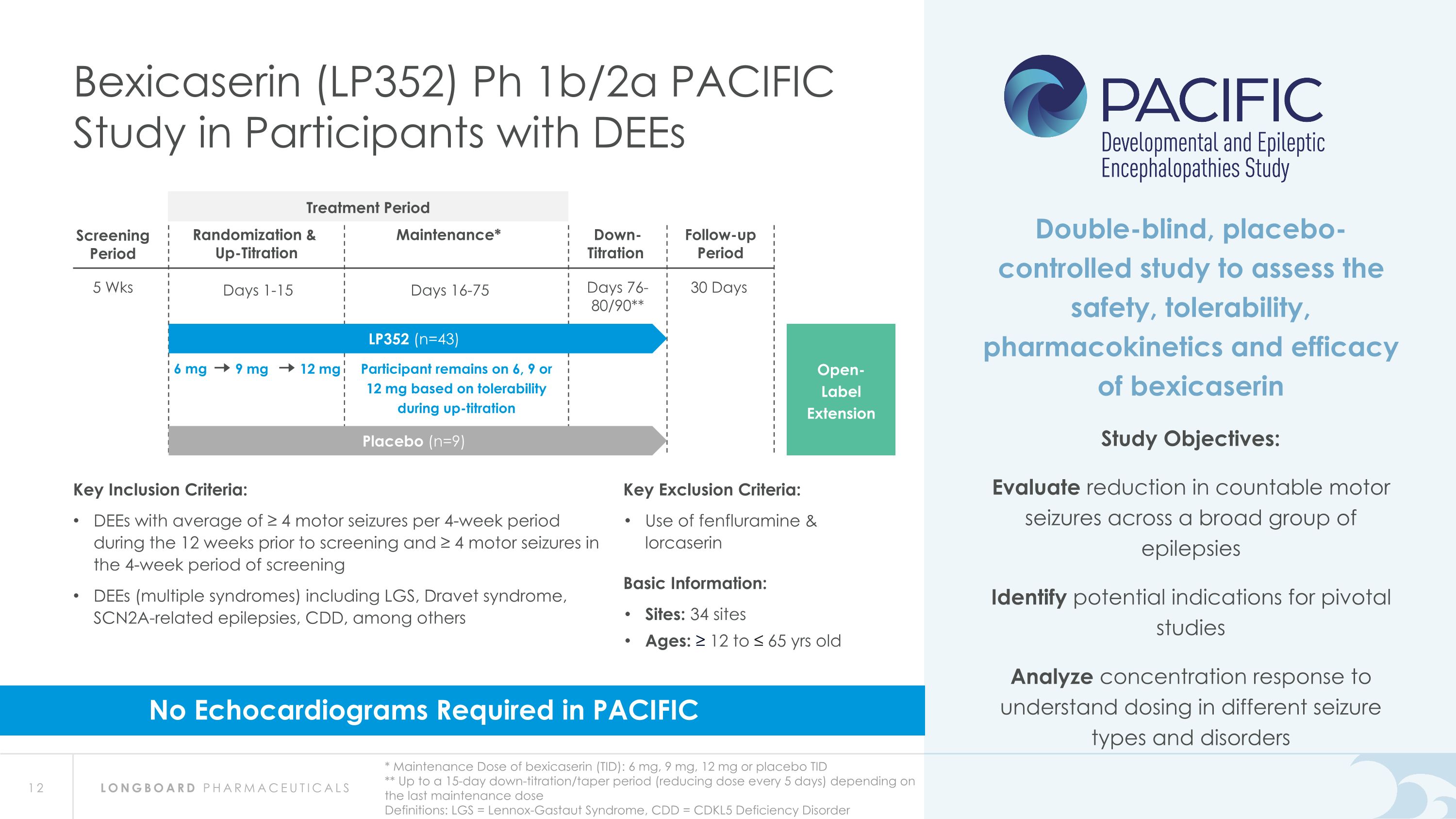
6 mg 9 mg 12 mg Participant remains on 6, 9 or 12 mg based on tolerability during up-titration 5 Wks Screening Period 30 Days Follow-up Period Randomization & Up-Titration Days 1-15 Maintenance* Days 16-75 Down- Titration Days 76- 80/90** Bexicaserin (LP352) Ph 1b/2a PACIFIC Study in Participants with DEEs * Maintenance Dose of bexicaserin (TID): 6 mg, 9 mg, 12 mg or placebo TID ** Up to a 15-day down-titration/taper period (reducing dose every 5 days) depending on the last maintenance dose Definitions: LGS = Lennox-Gastaut Syndrome, CDD = CDKL5 Deficiency Disorder Open- Label Extension Double-blind, placebo-controlled study to assess the safety, tolerability, pharmacokinetics and efficacy of bexicaserin Study Objectives: Evaluate reduction in countable motor seizures across a broad group of epilepsies Identify potential indications for pivotal studies Analyze concentration response to understand dosing in different seizure types and disorders Placebo (n=9) LP352 (n=43) Key Inclusion Criteria: DEEs with average of ≥ 4 motor seizures per 4-week period during the 12 weeks prior to screening and ≥ 4 motor seizures in the 4-week period of screening DEEs (multiple syndromes) including LGS, Dravet syndrome, SCN2A-related epilepsies, CDD, among others Key Exclusion Criteria: Use of fenfluramine & lorcaserin Basic Information: Sites: 34 sites Ages: ≥ 12 to ≤ 65 yrs old No Echocardiograms Required in PACIFIC Treatment Period
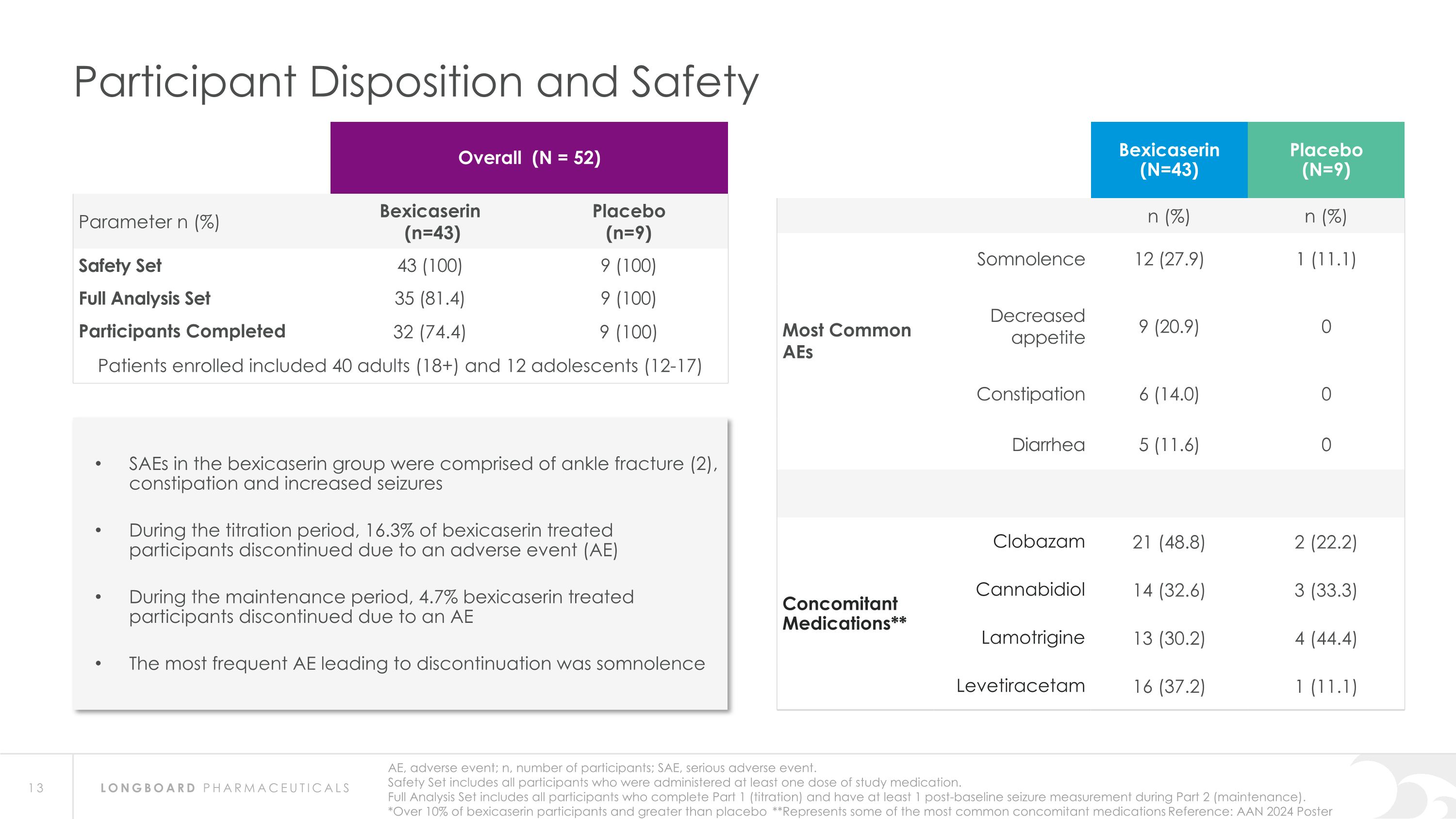
AE, adverse event; n, number of participants; SAE, serious adverse event. Safety Set includes all participants who were administered at least one dose of study medication. Full Analysis Set includes all participants who complete Part 1 (titration) and have at least 1 post-baseline seizure measurement during Part 2 (maintenance). *Over 10% of bexicaserin participants and greater than placebo **Represents some of the most common concomitant medications Reference: AAN 2024 Poster Participant Disposition and Safety Overall (N = 52) Parameter n (%) Bexicaserin (n=43) Placebo (n=9) Safety Set 43 (100) 9 (100) Full Analysis Set 35 (81.4) 9 (100) Participants Completed 32 (74.4) 9 (100) Patients enrolled included 40 adults (18+) and 12 adolescents (12-17) Bexicaserin (N=43) Placebo (N=9) n (%) n (%) Most Common AEs Somnolence 12 (27.9) 1 (11.1) Decreased appetite 9 (20.9) 0 Constipation 6 (14.0) 0 Diarrhea 5 (11.6) 0 Concomitant Medications** Clobazam 21 (48.8) 2 (22.2) Cannabidiol 14 (32.6) 3 (33.3) Lamotrigine 13 (30.2) 4 (44.4) Levetiracetam 16 (37.2) 1 (11.1) SAEs in the bexicaserin group were comprised of ankle fracture (2), constipation and increased seizures During the titration period, 16.3% of bexicaserin treated participants discontinued due to an adverse event (AE) During the maintenance period, 4.7% bexicaserin treated participants discontinued due to an AE The most frequent AE leading to discontinuation was somnolence
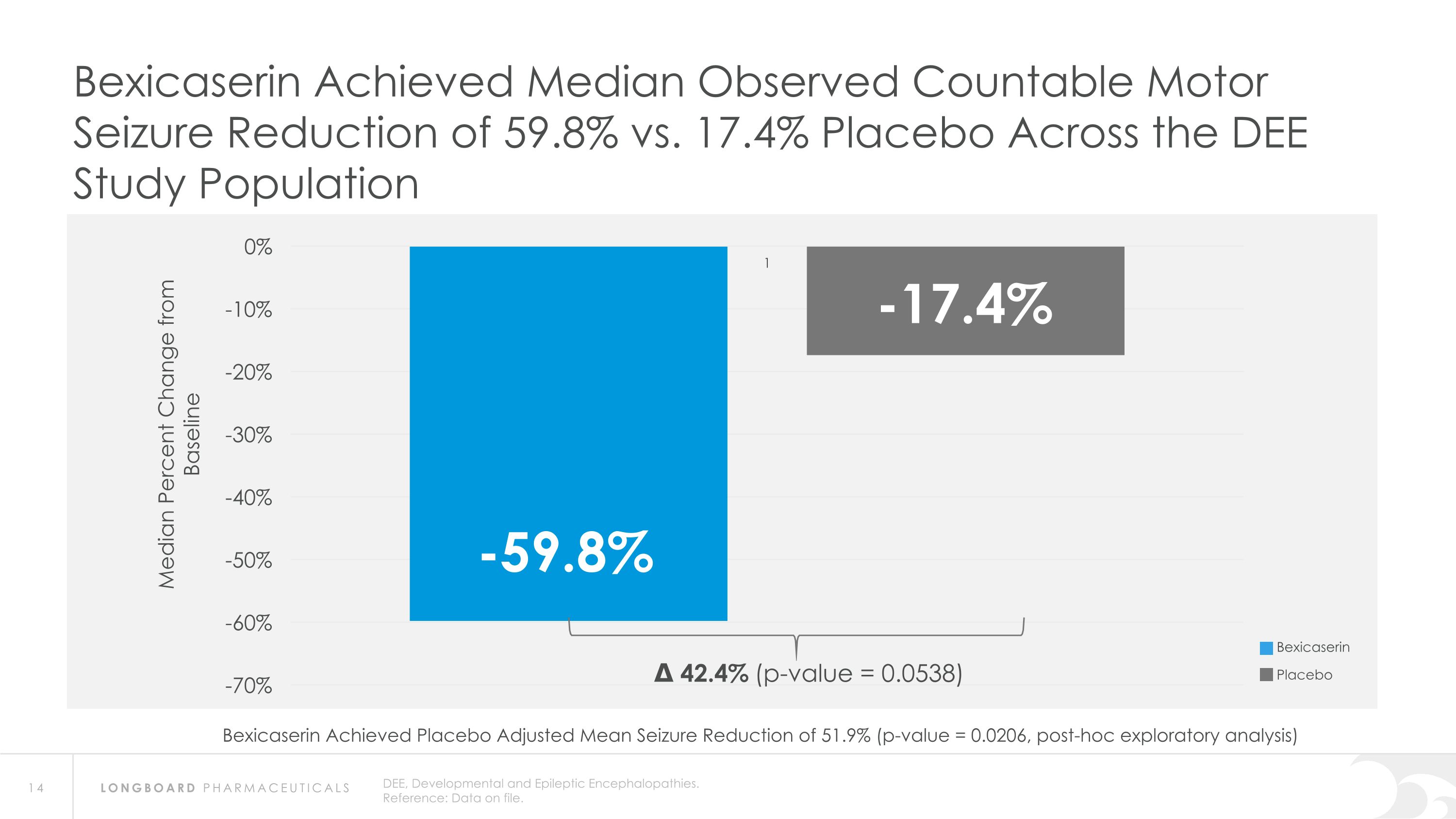
DEE, Developmental and Epileptic Encephalopathies. Reference: Data on file. Bexicaserin Achieved Median Observed Countable Motor Seizure Reduction of 59.8% vs. 17.4% Placebo Across the DEE Study Population Δ 42.4% (p-value = 0.0538) Bexicaserin Placebo Bexicaserin Achieved Placebo Adjusted Mean Seizure Reduction of 51.9% (p-value = 0.0206, post-hoc exploratory analysis)
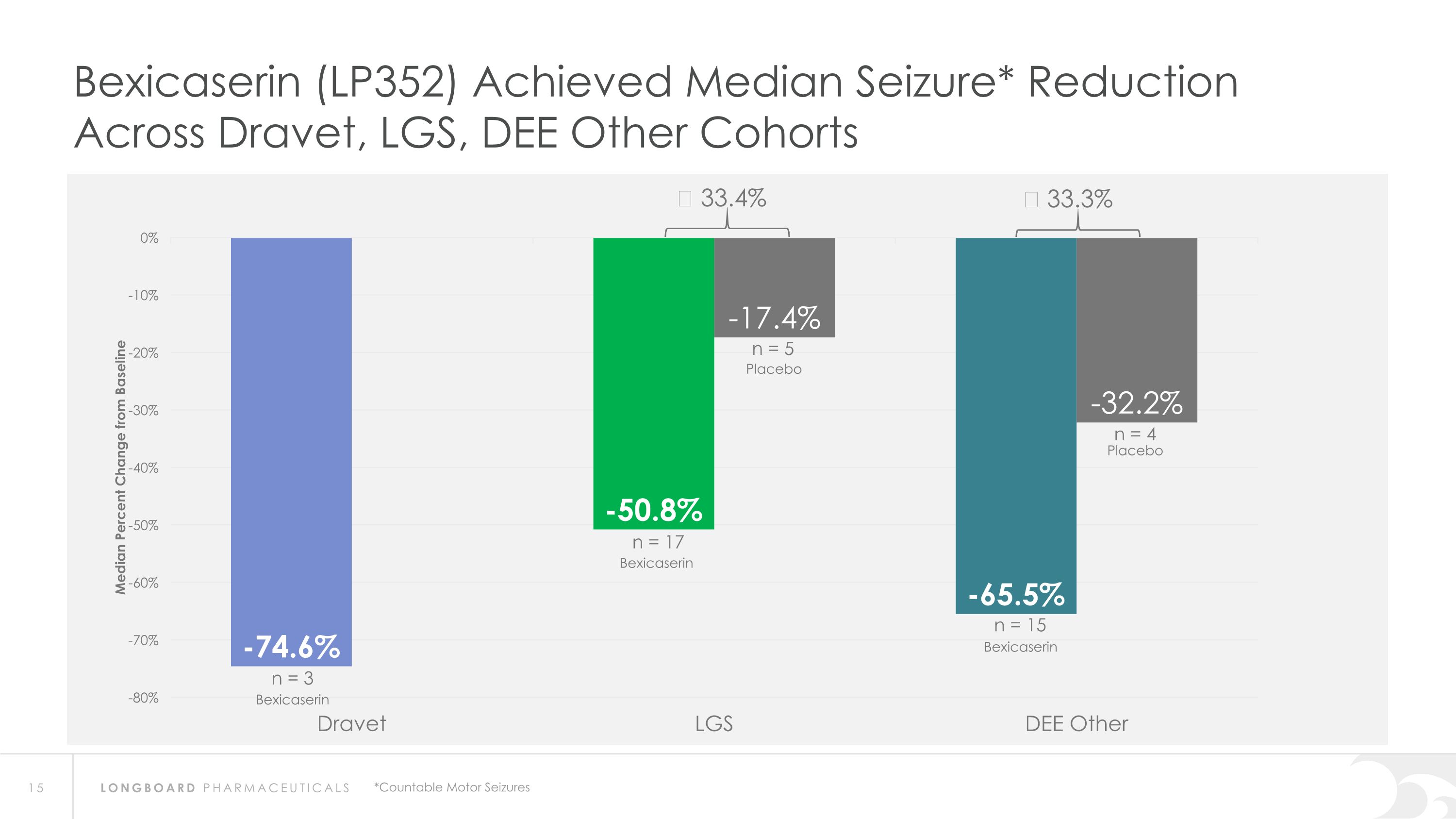
Bexicaserin (LP352) Achieved Median Seizure* Reduction Across Dravet, LGS, DEE Other Cohorts Δ 33.4% Δ 33.3% *Countable Motor Seizures n = 3 n = 5 n = 15 n = 4 Placebo Bexicaserin Placebo Bexicaserin Bexicaserin n = 17
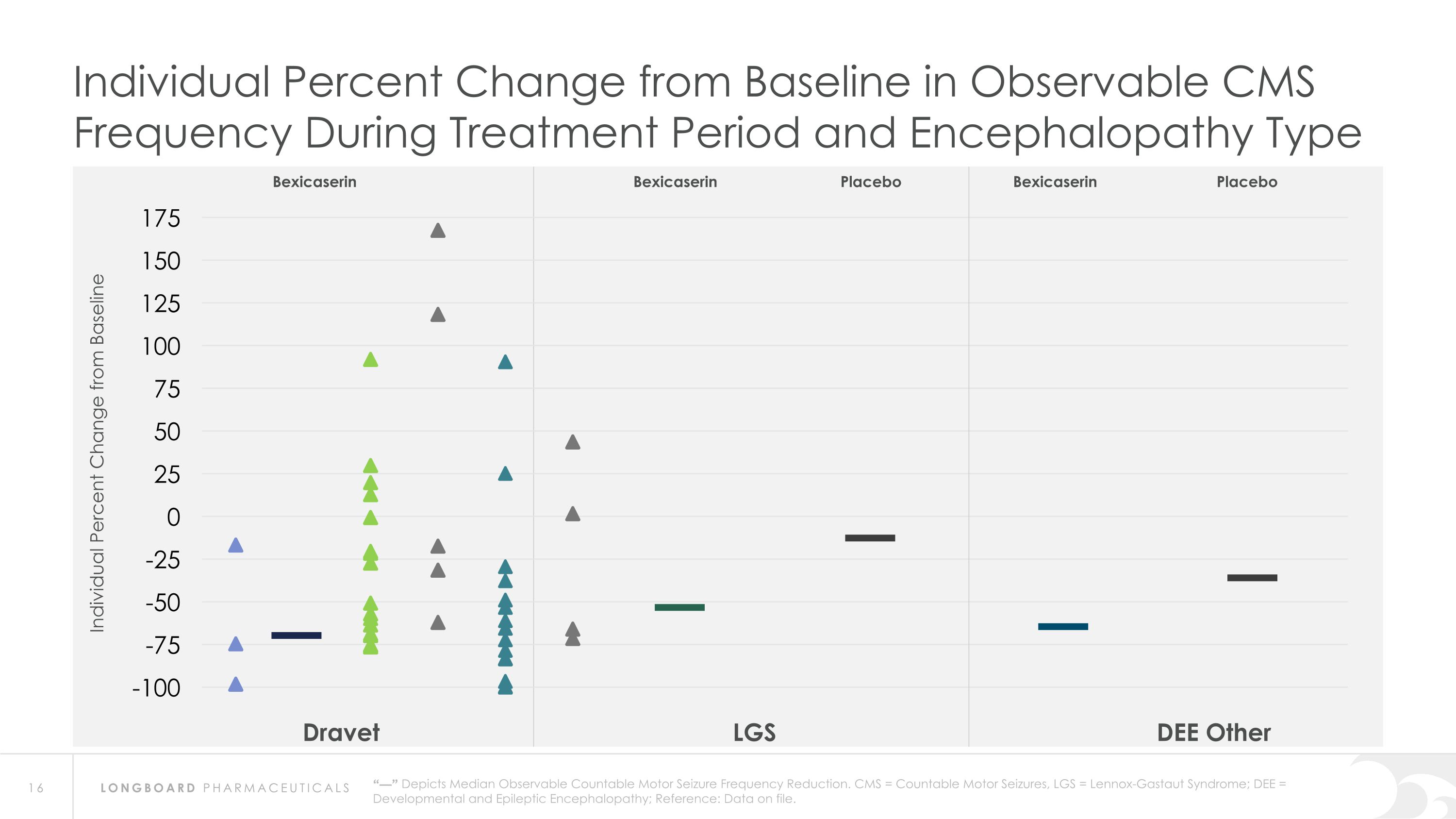
Dravet LGS DEE Other “—” Depicts Median Observable Countable Motor Seizure Frequency Reduction. CMS = Countable Motor Seizures, LGS = Lennox-Gastaut Syndrome; DEE = Developmental and Epileptic Encephalopathy; Reference: Data on file. Bexicaserin Placebo Bexicaserin Placebo Bexicaserin Individual Percent Change from Baseline in Observable CMS Frequency During Treatment Period and Encephalopathy Type
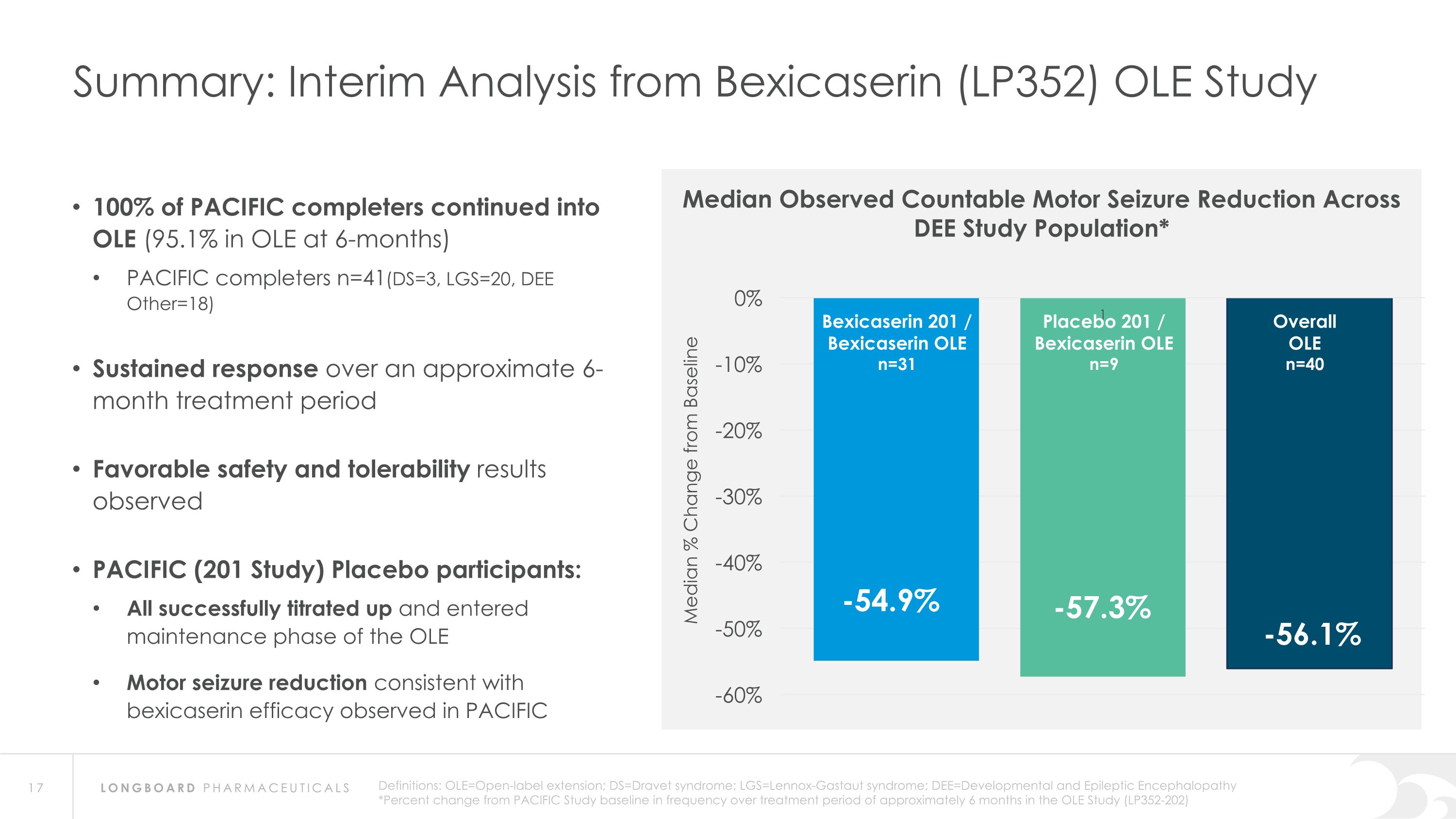
Summary: Interim Analysis from Bexicaserin (LP352) OLE Study Bexicaserin 201 / Bexicaserin OLE n=31 Placebo 201 / Bexicaserin OLE n=9 Overall OLE n=40 Median Observed Countable Motor Seizure Reduction Across DEE Study Population* 100% of PACIFIC completers continued into OLE (95.1% in OLE at 6-months) PACIFIC completers n=41(DS=3, LGS=20, DEE Other=18) Sustained response over an approximate 6-month treatment period Favorable safety and tolerability results observed PACIFIC (201 Study) Placebo participants: All successfully titrated up and entered maintenance phase of the OLE Motor seizure reduction consistent with bexicaserin efficacy observed in PACIFIC Definitions: OLE=Open-label extension; DS=Dravet syndrome; LGS=Lennox-Gastaut syndrome; DEE=Developmental and Epileptic Encephalopathy *Percent change from PACIFIC Study baseline in frequency over treatment period of approximately 6 months in the OLE Study (LP352-202)
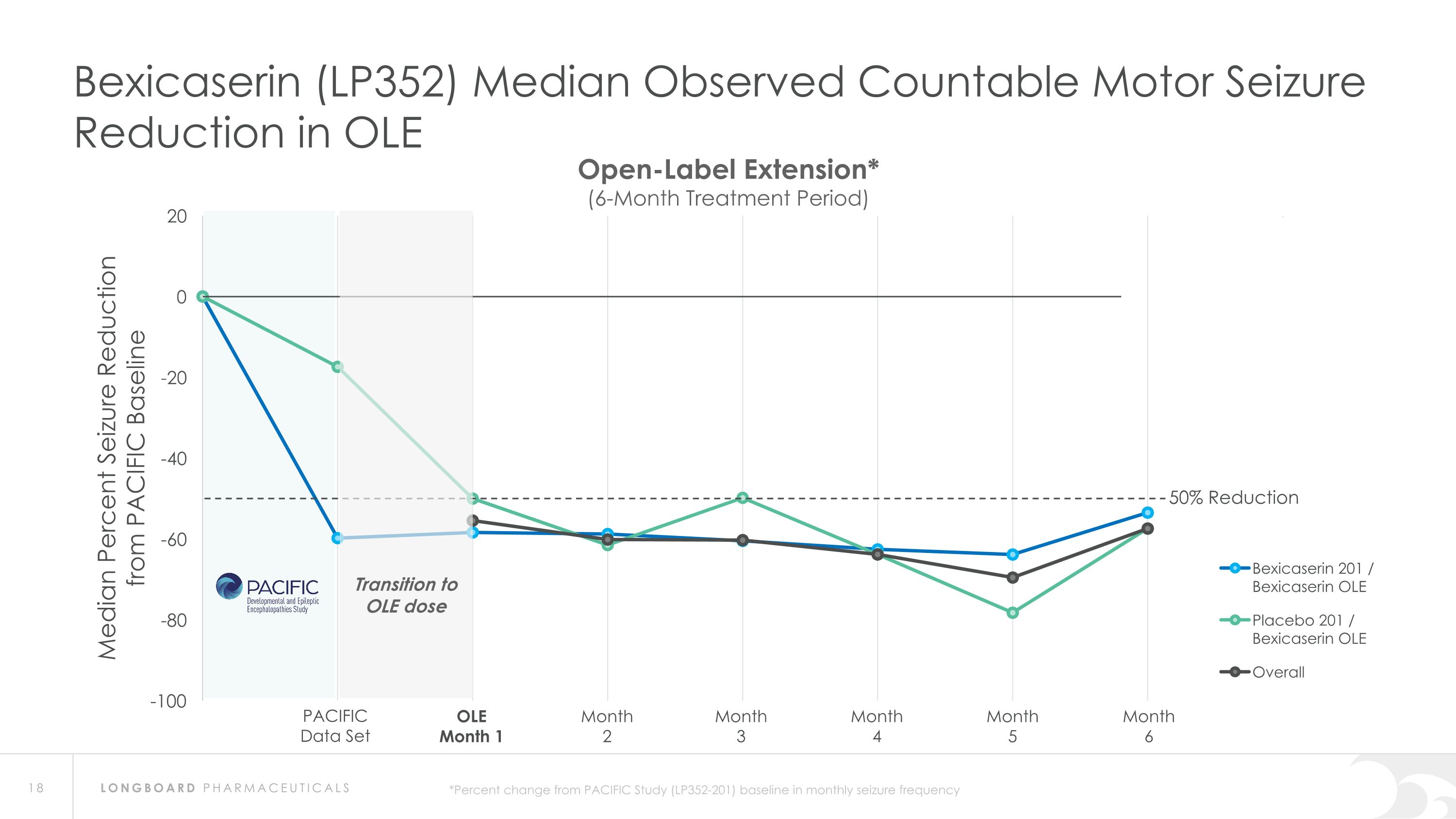
Bexicaserin (LP352) Median Observed Countable Motor Seizure Reduction in OLE PACIFIC Data Set OLE Month 1 Month 2 Month 3 Month 4 Month 5 Month 6 Open-Label Extension* (6-Month Treatment Period) 50% Reduction *Percent change from PACIFIC Study (LP352-201) baseline in monthly seizure frequency Transition to OLE dose

LGS = Lennox-Gastaut Syndrome Bexicaserin Phase 3 Global Program – DEE Path Forward Subject to Ongoing Discussions with Regulatory Agencies Planned Study Parameters: Primary Endpoint: Reduction in Countable Motor Seizures Ages: ≥ 2 to ≤ 65 yrs old (weight-based dosing for pts of lower weight/age) Sites: Sites across the US, AUS, EU, other potential regions Open-Label Extension (OLE): Participants who complete either of the Ph 3 studies are eligible to enter a 52-week OLE Study 302: Dravet Syndrome Study 301: DEEs (LGS + Other DEEs) Expected to be run in parallel Breakthrough Therapy designation granted for bexicaserin for the treatment of seizures associated with Developmental and Epileptic Encephalopathies (DEEs) for patients ≥ 2 years of age

Global Phase 3 Program Expected to Initiate in 2024 Bexicaserin (LP352) achieved a median percent reduction from baseline in seizure frequency during the treatment period of: 59.8% in broad DEE population (42.4% placebo-adjusted) 74.6% in Dravet cohort 50.8% in LGS cohort (33.4% placebo-adjusted) 65.5% in DEE Other cohort (33.3% placebo-adjusted) Results were shown on top of a contemporary polytherapy background with multiple ASMs including cannabidiol (32.7% of participants were receiving cannabidiol) *Definitions: ASMs = Anti-Seizure Medications; UGT = Uridine Diphosphate Glucuronosyltransferase Favorable safety and tolerability results No echocardiograms required in PACIFIC study Metabolized via UGT pathway – potentially reduces risk of Drug-Drug Interactions 86% of participants achieved the highest dose of 12 mg of bexicaserin in the maintenance period 100% of PACIFIC participants who completed the study entered the Open Label Extension (OLE) Study OLE Interim Analysis Sustained response over an approximate 6-month treatment period Favorable safety and tolerability results observed All PACIFIC Placebo participants successfully titrated up and entered maintenance phase of the OLE Motor seizure reduction consistent with bexicaserin efficacy observed in PACIFIC

LP659 Centrally Acting, Highly Selective Sphingosine-1-Phosphate (S1P) Receptor Modulator Targeting Multiple Neurological Diseases
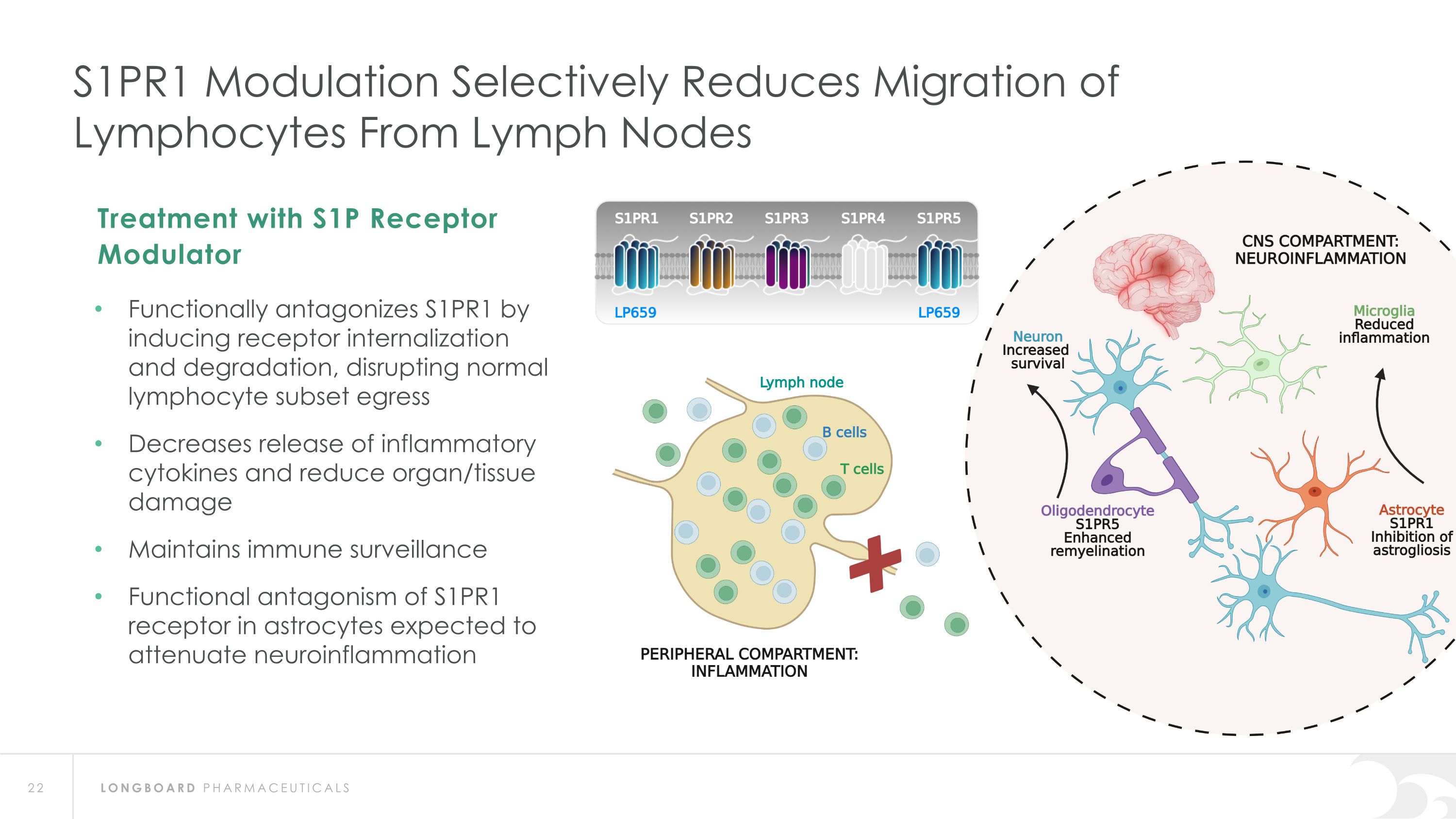
S1PR1 Modulation Selectively Reduces Migration of Lymphocytes From Lymph Nodes Functionally antagonizes S1PR1 by inducing receptor internalization and degradation, disrupting normal lymphocyte subset egress Decreases release of inflammatory cytokines and reduce organ/tissue damage Maintains immune surveillance Functional antagonism of S1PR1 receptor in astrocytes expected to attenuate neuroinflammation Treatment with S1P Receptor Modulator
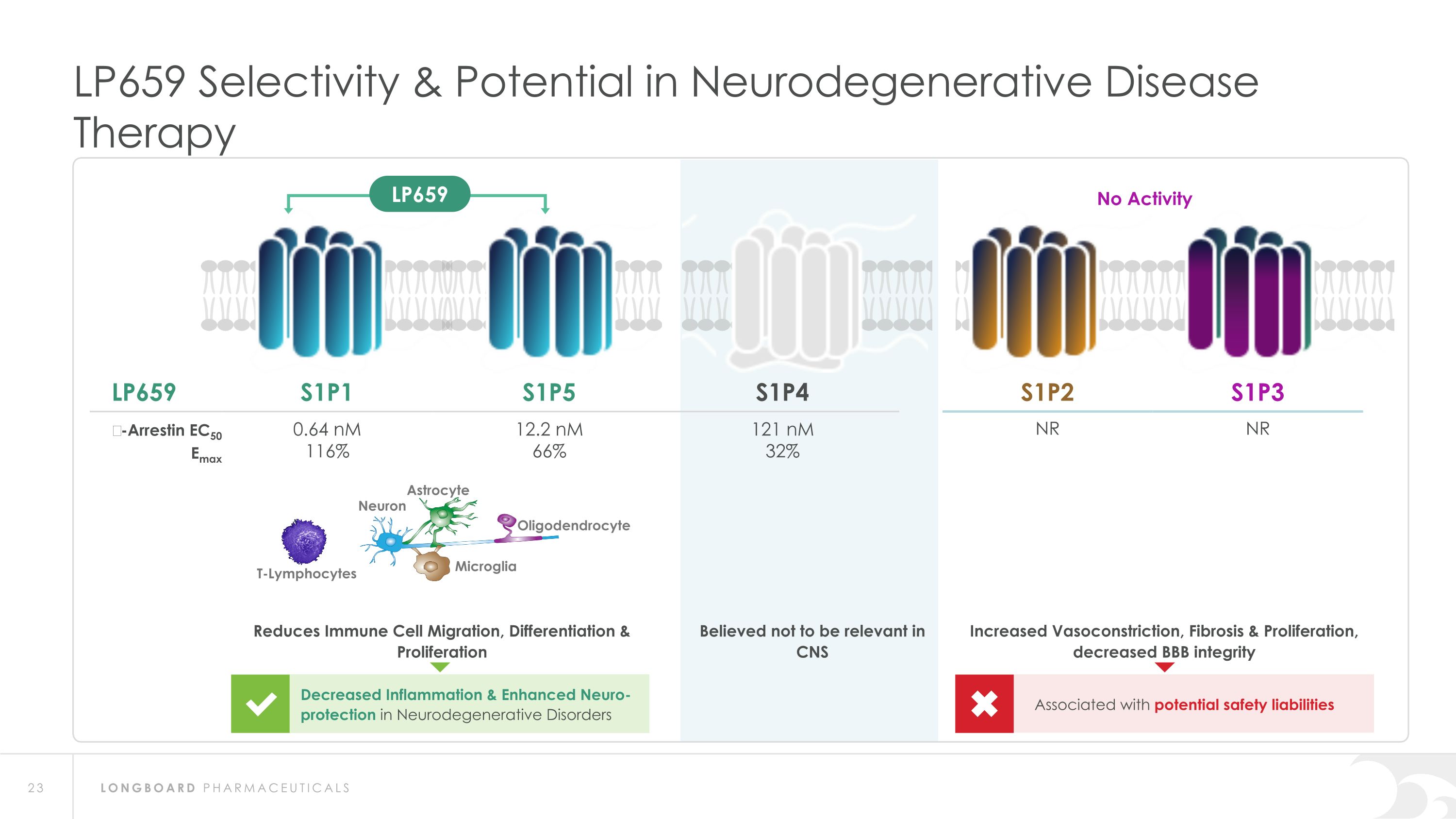
LP659 Selectivity & Potential in Neurodegenerative Disease Therapy LP659 S1P1 S1P5 S1P4 S1P2 S1P3 β-Arrestin EC50 Emax 0.64 nM 116% 12.2 nM 66% 121 nM 32% NR NR T-Lymphocytes Neuron Astrocyte Oligodendrocyte Microglia Decreased Inflammation & Enhanced Neuro-protection in Neurodegenerative Disorders Reduces Immune Cell Migration, Differentiation & Proliferation Associated with potential safety liabilities Increased Vasoconstriction, Fibrosis & Proliferation, decreased BBB integrity No Activity LP659 Believed not to be relevant in CNS
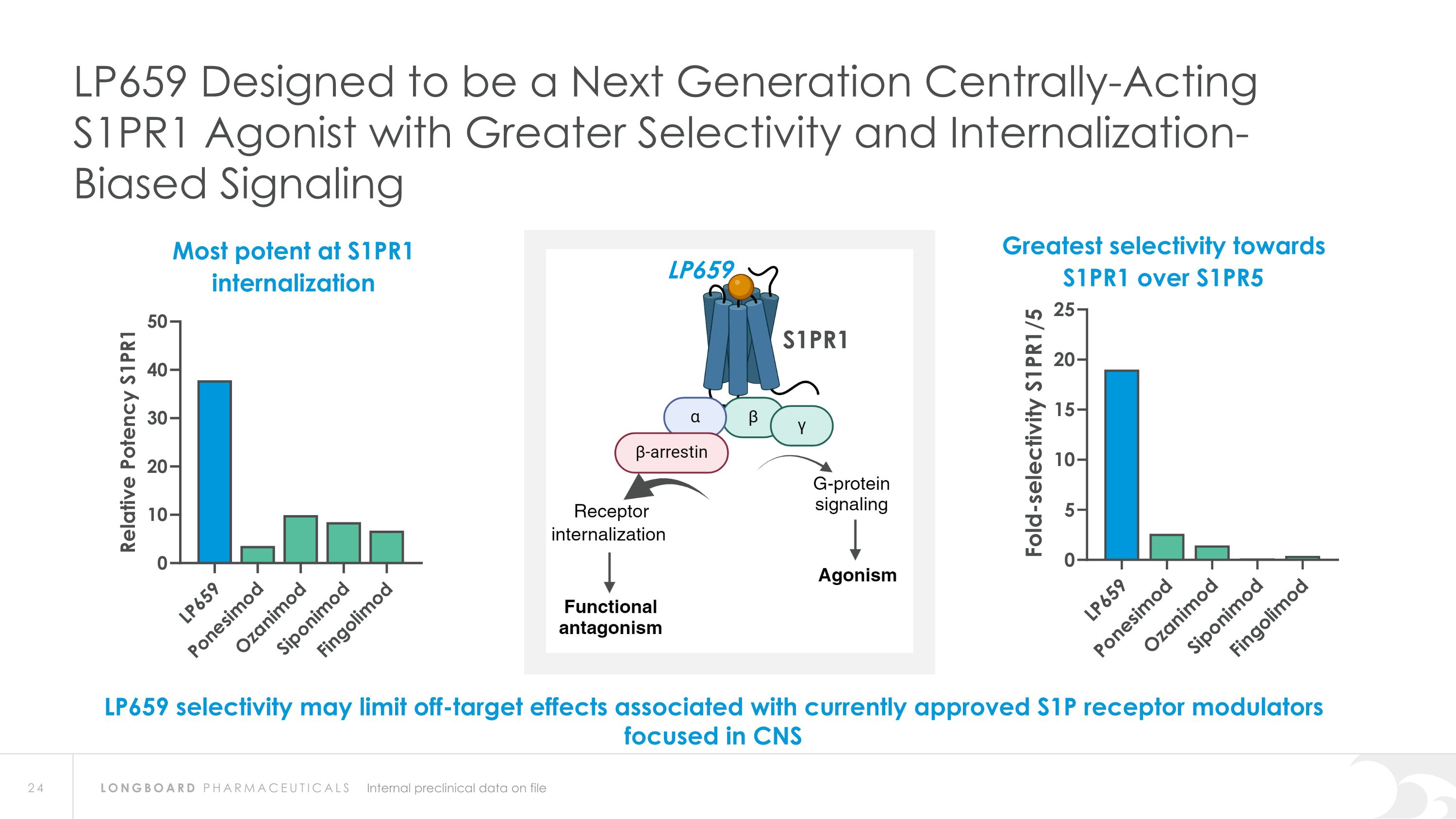
S1PR1 Internal preclinical data on file LP659 Designed to be a Next Generation Centrally-Acting S1PR1 Agonist with Greater Selectivity and Internalization-Biased Signaling LP659 selectivity may limit off-target effects associated with currently approved S1P receptor modulators focused in CNS Greatest selectivity towards S1PR1 over S1PR5 Most potent at S1PR1 internalization LP659
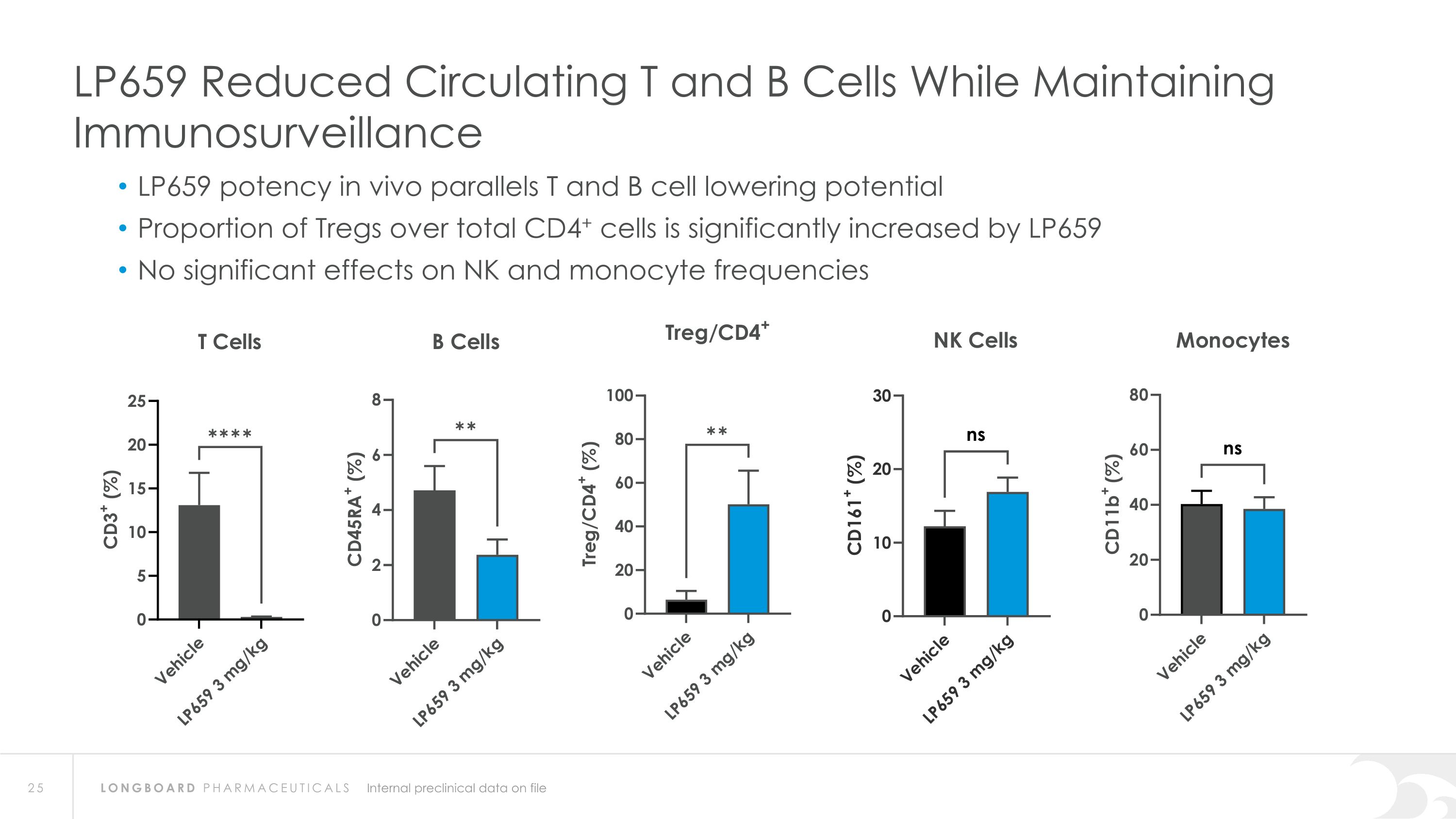
LP659 Reduced Circulating T and B Cells While Maintaining Immunosurveillance LP659 potency in vivo parallels T and B cell lowering potential Proportion of Tregs over total CD4+ cells is significantly increased by LP659 No significant effects on NK and monocyte frequencies Internal preclinical data on file

LP659 LP659 Phase 1 SAD Topline Data
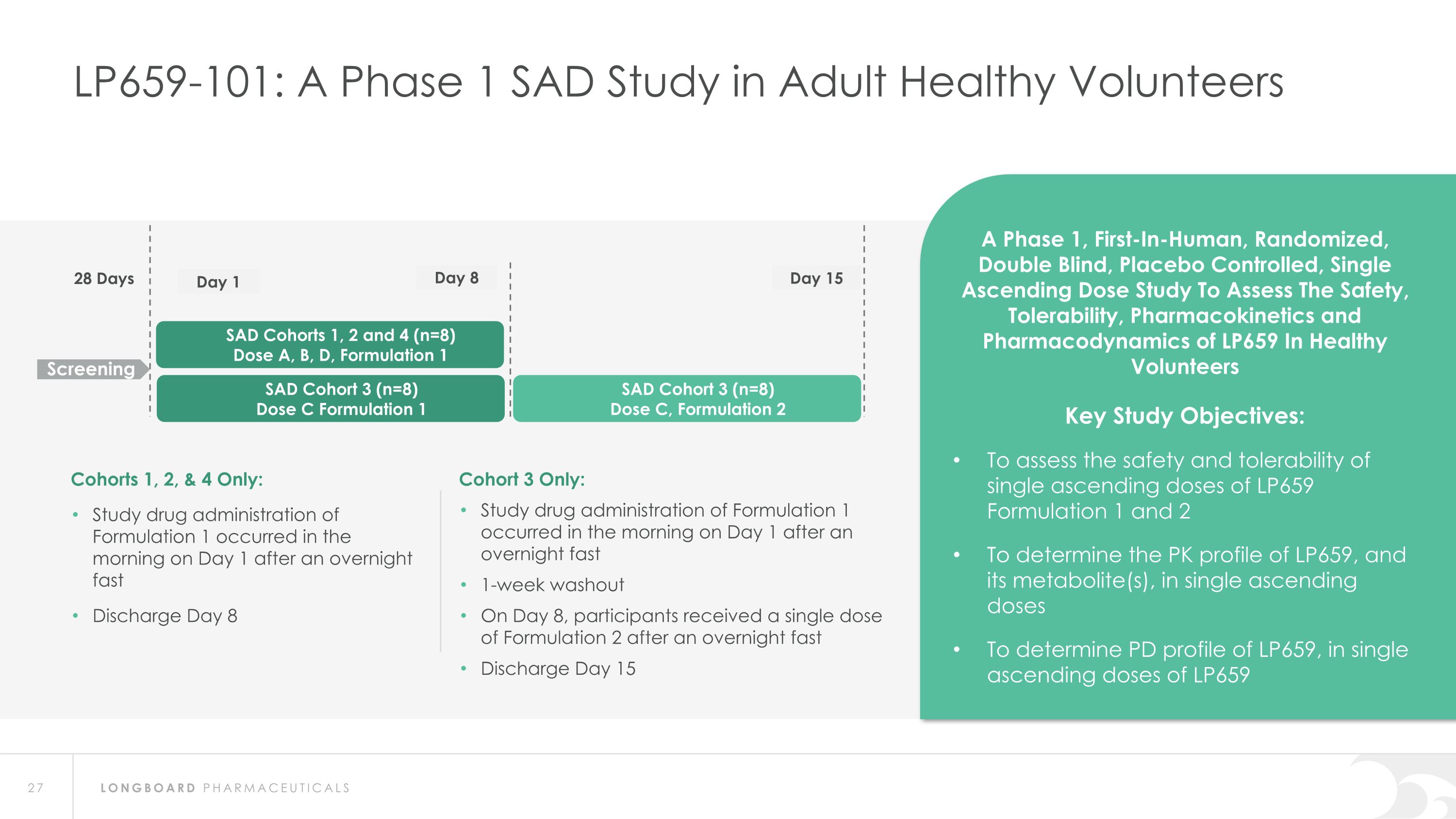
LP659-101: A Phase 1 SAD Study in Adult Healthy Volunteers SAD Cohorts 1, 2 and 4 (n=8) Dose A, B, D, Formulation 1 28 Days Screening A Phase 1, First-In-Human, Randomized, Double Blind, Placebo Controlled, Single Ascending Dose Study To Assess The Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of LP659 In Healthy Volunteers Key Study Objectives: To assess the safety and tolerability of single ascending doses of LP659 Formulation 1 and 2 To determine the PK profile of LP659, and its metabolite(s), in single ascending doses To determine PD profile of LP659, in single ascending doses of LP659 Day 1 Day 15 Cohorts 1, 2, & 4 Only: Study drug administration of Formulation 1 occurred in the morning on Day 1 after an overnight fast Discharge Day 8 Cohort 3 Only: Study drug administration of Formulation 1 occurred in the morning on Day 1 after an overnight fast 1-week washout On Day 8, participants received a single dose of Formulation 2 after an overnight fast Discharge Day 15 Day 8 SAD Cohort 3 (n=8) Dose C, Formulation 2 SAD Cohort 3 (n=8) Dose C Formulation 1

Adverse events were mild No TEAEs leading to discontinuation No SAEs observed Impact on HR was low throughout the study with no first dose bradycardia No abnormal ECGs (no AV block) or abnormal echocardiograms No abnormal pulmonary/spirometry and ophthalmologic assessments No infections Phase 1 Safety Results LP659 was generally safe and well tolerated ► Abbreviations: TEAEs = Treatment-Emergent Adverse Events; SAEs = serious adverse events, HR = Heart Rate, ECG = electrocardiogram, AV = atrioventricular
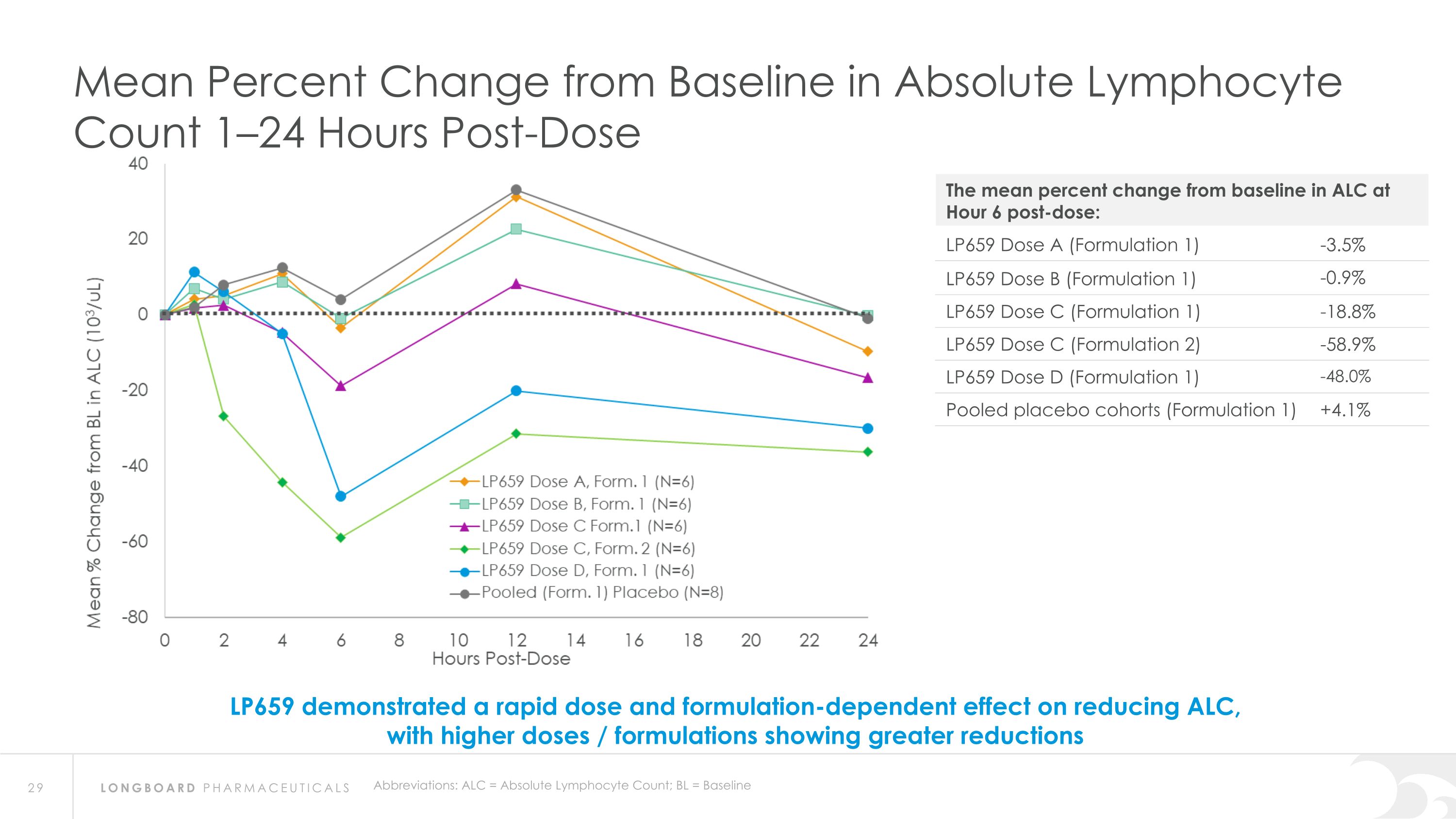
Abbreviations: ALC = Absolute Lymphocyte Count; BL = Baseline Mean Percent Change from Baseline in Absolute Lymphocyte Count 1–24 Hours Post-Dose LP659 demonstrated a rapid dose and formulation-dependent effect on reducing ALC, with higher doses / formulations showing greater reductions The mean percent change from baseline in ALC at Hour 6 post-dose: LP659 Dose A (Formulation 1) -3.5% LP659 Dose B (Formulation 1) -0.9% LP659 Dose C (Formulation 1) -18.8% LP659 Dose C (Formulation 2) -58.9% LP659 Dose D (Formulation 1) -48.0% Pooled placebo cohorts (Formulation 1) +4.1%
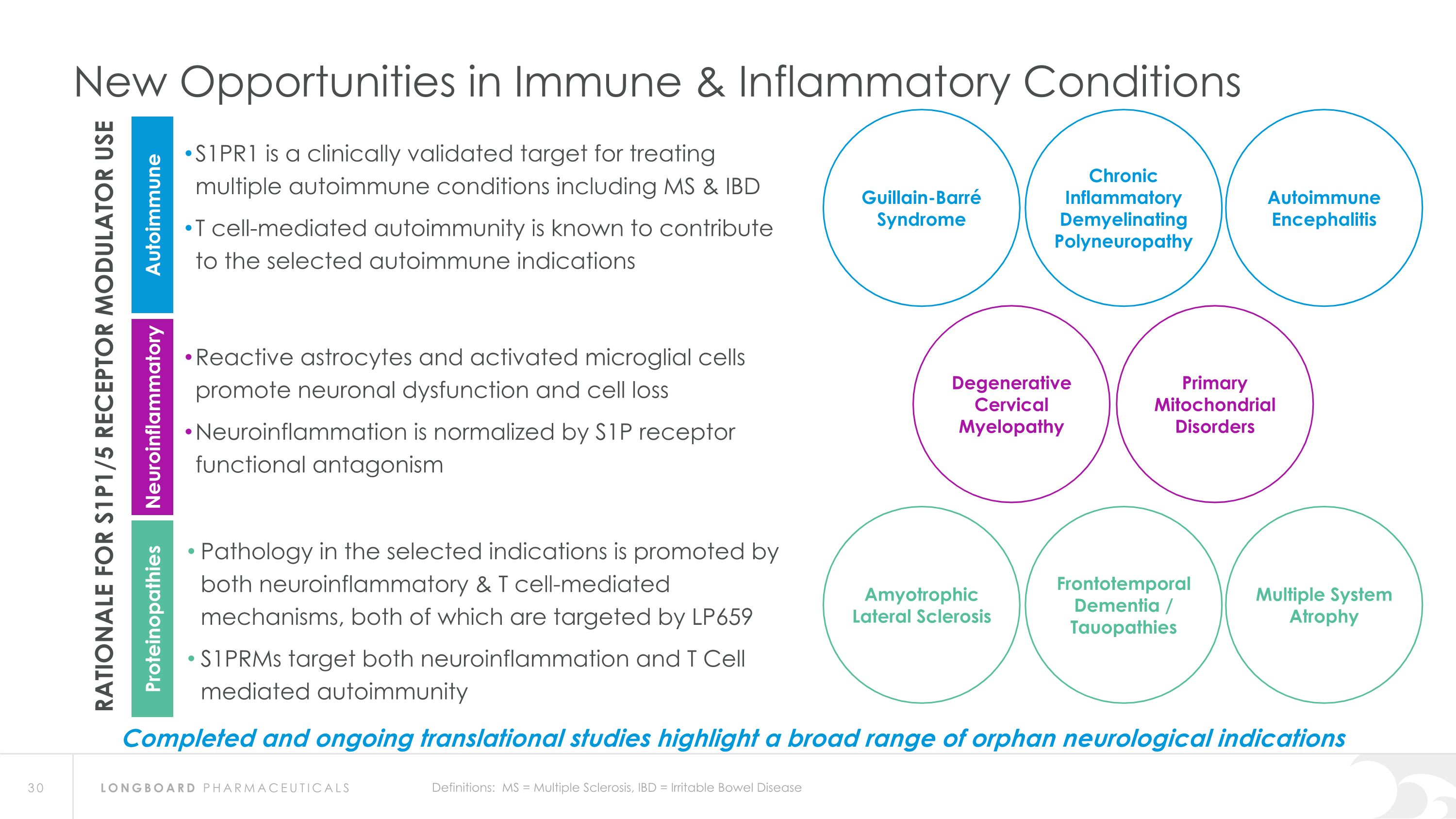
New Opportunities in Immune & Inflammatory Conditions S1PR1 is a clinically validated target for treating multiple autoimmune conditions including MS & IBD T cell-mediated autoimmunity is known to contribute to the selected autoimmune indications RATIONALE FOR S1P1/5 RECEPTOR MODULATOR USE Autoimmune Neuroinflammatory Proteinopathies Reactive astrocytes and activated microglial cells promote neuronal dysfunction and cell loss Neuroinflammation is normalized by S1P receptor functional antagonism Pathology in the selected indications is promoted by both neuroinflammatory & T cell-mediated mechanisms, both of which are targeted by LP659 S1PRMs target both neuroinflammation and T Cell mediated autoimmunity Degenerative Cervical Myelopathy Primary Mitochondrial Disorders Autoimmune Encephalitis Guillain-Barré Syndrome Chronic Inflammatory Demyelinating Polyneuropathy Definitions: MS = Multiple Sclerosis, IBD = Irritable Bowel Disease Multiple System Atrophy Amyotrophic Lateral Sclerosis Frontotemporal Dementia / Tauopathies Completed and ongoing translational studies highlight a broad range of orphan neurological indications
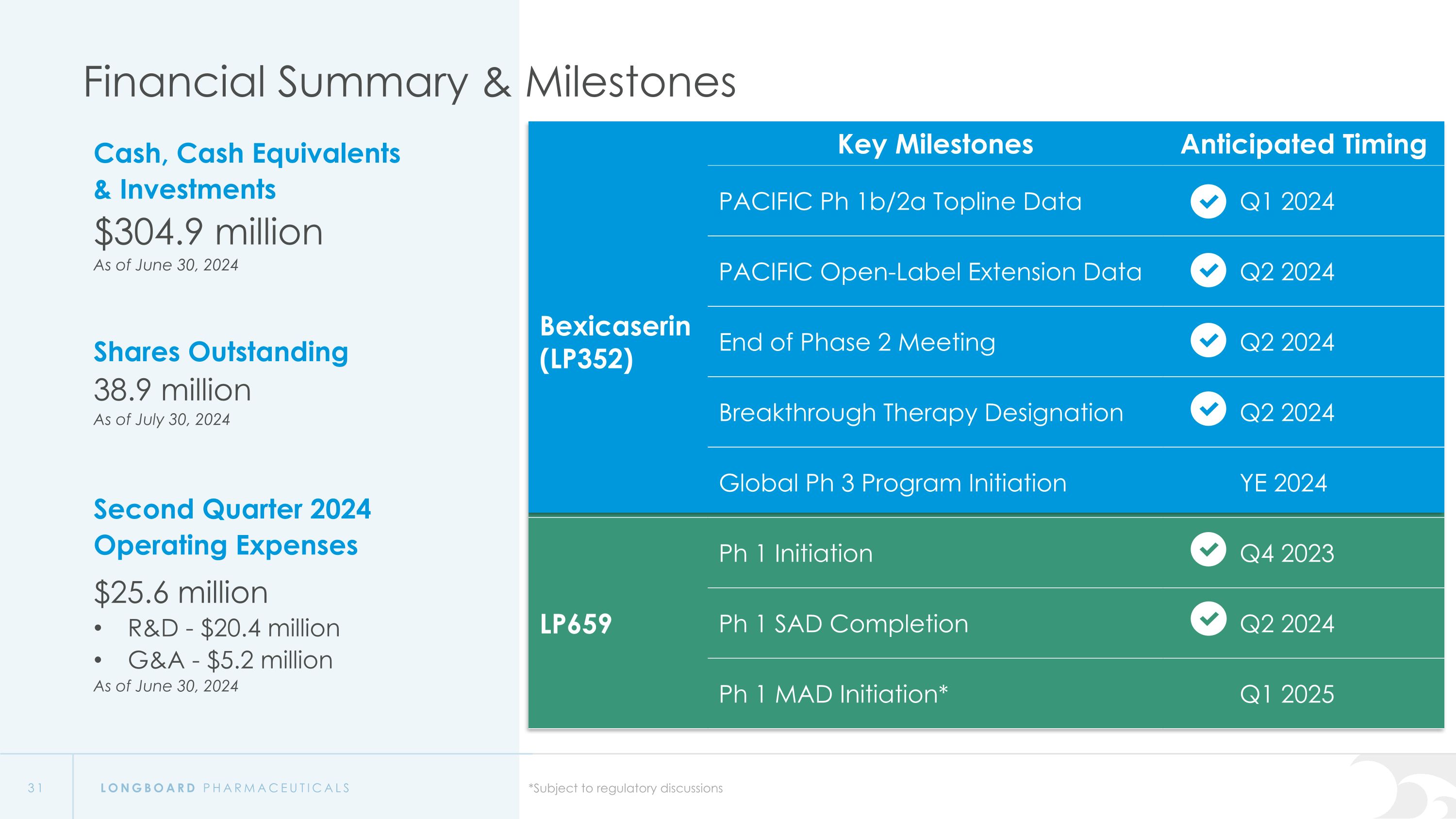
Financial Summary & Milestones Cash, Cash Equivalents & Investments $304.9 million As of June 30, 2024 Shares Outstanding 38.9 million As of July 30, 2024 Second Quarter 2024 Operating Expenses $25.6 million R&D - $20.4 million G&A - $5.2 million As of June 30, 2024 Key Milestones Anticipated Timing Bexicaserin (LP352) PACIFIC Ph 1b/2a Topline Data Q1 2024 PACIFIC Open-Label Extension Data Q2 2024 LP352 End of Phase 2 Meeting Q2 2024 Breakthrough Therapy Designation Q2 2024 Global Ph 3 Program Initiation YE 2024 LP659 Ph 1 Initiation Q4 2023 Ph 1 SAD Completion Q2 2024 Ph 1 MAD Initiation* Q1 2025 *Subject to regulatory discussions

Thank you Nasdaq: LBPH IR@longboardpharma.com