① Huadong Pharmaceutical achieved revenue of 20.965 billion yuan, net profit of 1.696 billion yuan in the first half of the year, and a planned mid-term dividend of about 0.614 billion yuan. ② Huadong Pharmaceutical is speeding up the deployment of innovative drugs in the three major treatment fields of endocrinology, autoimmunity, and oncology. It is expected that 5 innovative products will be approved for launch in the second half of 2024.
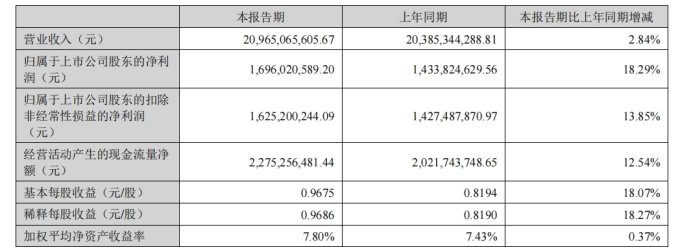
“Science and Technology Innovation Board Daily”, August 15 (Reporter Zheng Bingxun) This evening, Huadong Pharmaceutical (000963.SZ) released its 2024 semi-annual report, ** achieving revenue of 20.965 billion yuan, up 2.84% year on year, and net profit of 1.696 billion yuan to mother, up 18.29% year on year.
**Among them, the second quarter achieved revenue of 10.554 billion yuan, a year-on-year increase of 2.76%, and net profit to mother of 0.834 billion yuan, an increase of 22.85% year-on-year.
At the same time as announcing the results, Huadong Pharmaceutical also announced a semi-annual profit distribution plan. It plans to pay a mid-term dividend of 3.5 yuan for every 10 shares, with a cumulative distribution of about 0.614 billion yuan.
Currently, Huadong Pharmaceutical has four major business segments: pharmaceutical industry, pharmaceutical commerce, medical aesthetics, and industrial microbiology. Currently, the pharmaceutical business is still Huadong Pharmaceutical's largest source of revenue. In the first half of the year, the pharmaceutical business sector achieved revenue of 13.552 billion yuan, a year-on-year decrease of 0.58%, and achieved cumulative net profit of 0.218 billion yuan, an increase of 0.90% over the previous year.
In contrast, the pharmaceutical industry is becoming a sector of rapid development in East China Pharmaceuticals. In the first half of the year, the pharmaceutical industry achieved operating income of 6.698 billion yuan (including CSO business), an increase of 10.63% over the previous year, and a net profit of 1.385 billion yuan to mother, an increase of 11.48% over the previous year.
Huadong Pharmaceutical's innovative drug layout in the three major treatment fields of endocrinology, autoimmunity, and oncology is gradually showing results. According to its introduction, 5 innovative products are expected to be approved for launch in the second half of 2024.
Specifically, in the field of oncology, the world's first new ADC drug, somituximab injection (ELAHERE), introduced by Huadong Pharmaceutical, has been approved for marketing in China for platinum-resistant ovarian cancer. The product was previously approved for marketing in Macau in April of this year. Furthermore, Huadong Pharmaceutical reached an exclusive marketing cooperation with Yingpai Pharmaceutical on the innovative PARP inhibitor Senapalil in December last year. Senapali's application for listing in China was accepted in August last year.
It is worth mentioning that Huadong Pharmaceutical moves frequently on the CAR-T circuit.
First, in January of last year, Huadong Pharmaceutical signed an exclusive cooperation agreement with Kaixing Life to obtain the other party's exclusive commercial rights for the CAR-T product Zewokie Orense Injection (trade name; Cykaize) for the treatment of recurrent and difficult to treat multiple myeloma in mainland China. The product was approved for marketing in March of this year. By the end of July 2024, 129 medical institutions had received training and certification to use Sekaiser. In the preliminary review list for 2024 health insurance negotiations announced a few days ago, Zewokie Orense injection is on the list.
Second, in August of this year, Huadong Pharmaceutical and Yimiao Shenzhou reached a cooperation to obtain exclusive commercial rights for IM19 injection, an autologous CAR-T candidate product targeting CD19, in mainland China.
In the field of self-immunity, Huadong Pharmaceutical and Quanxin Biotech's cooperation application for the marketing license of the usinumab biosimilar HDM3001 (QX001S) in China were accepted in August 2023 for the treatment of plaque psoriasis and is currently under review.
At the same time, the Chinese marketing application for the innovative drug ARCALYST (injectable linacip) introduced by Huadong Pharmaceutical to treat cyclic syndrome and recurrent pericarditis associated with cold pyrine has also been accepted. Both indications are varieties on the national rare disease catalogue, and both have been included in the list of priority review varieties.
However, at the same time as Huadong Pharmaceutical released its semi-annual report, it was also announced that its wholly-owned subsidiary China and US Huadong decided to stop follow-up research and development of the TTP273 project. This product was introduced by China and US Huadong from the US vTV company at the end of 2017.
Regarding the termination of TTP273, Huadong Pharmaceutical explained that TTP273 and the self-developed HDM1002 are both oral small molecule GLP-1 receptor agonists. Current data shows that HDM1002 is superior to TTP273 in terms of efficacy, activity, bioavailability, production difficulty and cost, and has more significant curative effects in weight loss indications and has higher development potential.
Up to now, Huadong Pharmaceutical's TTP273 project has accumulated a total direct R&D investment of 0.197 billion yuan, including a down payment of 10 million US dollars in license agreements and registration milestone payments.
Huadong Pharmaceutical revealed that as of August 2024, HDM1002 has completed all Phase II clinical studies of weight management indications for overweight or obese people, and is expected to obtain top-line results in Q4 2024.
