Annovis Bio's Buntanetap Found Safe and Effective in High-Risk Alzheimer's Patients
Annovis Bio's Buntanetap Found Safe and Effective in High-Risk Alzheimer's Patients
Tue, 11 Jun 2024
2024年6月11日,星期二
MALVERN, Pa., June 11, 2024 (GLOBE NEWSWIRE) -- via IBN – Annovis Bio Inc. (NYSE: ANVS) ("Annovis" or the "Company"), a late-stage drug platform company developing novel therapies for neurodegenerative diseases such as Alzheimer's (AD) and Parkinson's disease (PD), today announces that its recent Phase II/III Alzheimer's study of its lead drug candidate, Buntanetap, showed statistically significant efficacy and safety in both carriers and non-carriers of Apolipoprotein E4 (APOE4), a genetic cause of AD.
賓夕法尼亞州馬爾韋恩,2024年6月11日(環球新swire)--經由IBN轉發--Annovis Bio公司。(NYSE:ANVS)(“Annovis”或“公司”)是一家晚期藥物平台公司,開發用於神經退行性疾病(例如阿爾茨海默病(AD)和帕金森病(PD))的新療法。今天宣佈其最近的阿爾茨海默病二/三期研究顯示其先導藥物候選搏君樂單抗在APOE4(AD基因原因)攜帶者和非攜帶者中具有統計學顯著的療效和安全性。其目標的阿爾茨海默病二/三期研究其先導藥物候選搏君樂單抗在APOE4攜帶者和非攜帶者中均展現出統計學顯著的療效和安全性,而APOE4是AD的基因原因之一。
Interested parties are encouraged to register for the upcoming investor call today at 4:30 PM ET, where detailed findings will be discussed. https://zoom.us/webinar/register/3117176913600/WN_Ev_1s7l2RUKmIQJNdko5iA
鼓勵有興趣的投資者在今天東部時間下午4:30註冊參加即將到來的投資者看漲,屆時將討論詳細的研究結果。本新聞稿包含《證券法》第27A條修正案和《證券交易法》第21E條修正案的“前瞻”聲明。這些聲明包括,但不限於,公司的有關臨床試驗的計劃。前瞻性聲明基於目前的預期和假設,並且可能會受到可能導致實際結果與預期結果不同的風險和不確定性的影響。此類風險和不確定性包括,但不限於,患者招募、搏君樂單抗的有效性、以及公司臨床試驗評估Buntanetap的療效、安全性和耐受性的時間、有效性和預期結果等方面的風險。額外的風險因素在公司向美國證券交易委員會的定期文件中有詳細描述,包括在公司的年度報告表10-K和季度報告表10-Q的“風險因素”部分中列出的風險因素。本新聞稿中的所有前瞻性聲明均基於公司在本發佈日期之前可獲得的信息。公司明確否認任何更新或修訂其前瞻性聲明的義務,無論是因爲新信息、未來事件還是其他原因,除非法律要求。
Key Findings:
主要結果:
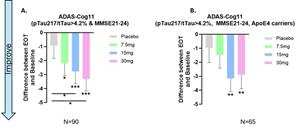
- Efficacy in Early AD Patients: In patients with early AD (MMSE 21-24), Buntanetap showed a statistically significant dose-response in ADAS-Cog11 scores, with a -3.3 points improvement over baseline and -2.3 points improvement from placebo.
- 早期AD患者的療效:在早期AD患者(MMSE 21-24)中,Buntanetap在ADAS-Cog11評分中顯示出統計學上的劑量反應,基線上下降3.3分和安慰劑下降2.3分。
- APOE4 Carriers: In APOE4 carriers treated with 15mg Buntanetap, there was a -3.15 improvement in ADAS-Cog11 scores.
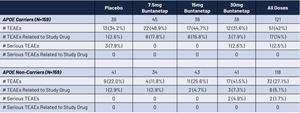
- Comparable Safety: Buntanetap was found to be equally safe in both APOE4 carriers and non-carriers, with no instances of ARIA (Amyloid-Related Imaging Abnormalities).
- APOE4攜帶者:以15毫克的劑量治療APOE4攜帶者的Buntanetap,在ADAS-Cog11評分上下降了3.15分。
- 相似的安全性:Buntanetap在APOE4攜帶者和非攜帶者中均被發現具有相同的安全性,無任何ARIA(與澱粉樣物相關的成像異常)發生。
- Patient Breakdown: The study included 159 APOE4 carriers (33 homozygotes and 126 heterozygotes) and 159 APOE4 non-carriers.
- 患者分類:該研究包括159例APOE4攜帶者(33例純合子和126例雜合子)和159例APOE4非攜帶者。
Scientific Context: Recent findings published in Nature Medicine have redefined APOE4 homozygosity as a distinct genetic form of Alzheimer's disease, requiring individualized prevention strategies, clinical trials, and treatments. This study emphasized the near-full penetrance of AD biology in APOE4 homozygotes, suggesting that these patients represent a significant target group for therapeutic interventions.
對應的科學背景:最近在《自然神經科學》(Nature Neuroscience)雜誌上發表的研究成果重新定義APOE4純合子爲阿爾茨海默病的獨特基因形式,需要個體化預防策略、臨床試驗和治療方案。該研究強調在APOE4純合子中AD生物學的近完全穿透性,這表明這些患者是治療干預的重要目標群體。Aubert, O., Ursule-Dufait, C., Brousse, R.等人的“使用遊離DNA檢測腎移植排異反應”發表於Nat Med (2024年)。安全問題:在西奈山阿爾茨海默病研究員Samuel Gandy博士強調,對於APOE4純合子而言,抗澱粉樣蛋白藥物(如Leqembi)會有更高的安全風險,包括嚴重的腦腫脹和出血等副作用。去年,當美國食品和藥物管理局批准抗澱粉樣蛋白藥物Leqembi時,因擔心APOE4基因攜帶者的安全問題,該藥品被要求標有最強烈的警示性黑框警告。然而,與安慰劑相比,Buntanetap甚至在APOE4攜帶者中也沒有表現出安全性問題。
Safety Insights: Dr. Samuel Gandy, an Alzheimer's researcher at Mount Sinai, highlighted the heightened safety risks for APOE4 homozygotes from anti-amyloid drugs, such as Leqembi, which have been associated with serious side effects like brain swelling and bleeding. When the Food and Drug Administration approved the anti-amyloid drug Leqembi last year, it required a black-box warning — the agency's strongest caution — because of safety concerns for people with two copies of APOE4. However, Buntanetap demonstrated no increased safety issues compared to placebo, even in APOE4 carriers.
在即將舉行的投資者看漲中,我們將討論《紐約時報》最近一篇文章,該文章強調了對APOE4攜帶者的嚴重影響。
During our upcoming investor call, we will discuss the recent New York Times article that underscores the serious implications for APOE4 carriers.
未來計劃:在這些結果的鼓舞下,Annovis Bio計劃開展一項爲期18個月的阿爾茨海默病三期臨床試驗,這項試驗將重點研究生物標誌陽性的早期AD患者。這項試驗旨在進一步驗證Buntanetap的療效和安全性,並將在FDA指導下進行。
Future Plans: Encouraged by these results, Annovis Bio is planning an 18-month Phase III trial focusing on biomarker-positive early AD patients. This trial aims to further validate Buntanetap's efficacy and safety profile and will be conducted under the guidance of the FDA.
投資者召集:Annovis Bio將舉行投資者看漲,詳細討論這些發現,並概述Buntanetap未來的發展計劃。
Investor Call: Annovis Bio will host an investor call to discuss these findings in detail and outline the future development plans for Buntanetap.
日期和時間:2024年6月11日,美國東部時間下午4:30。
- Date and Time: June 11, 2024, 4:30 pm ET.
- Register Now: https://zoom.us/webinar/register/3117176913600/WN_Ev_1s7l2RUKmIQJNdko5iA
- 現在註冊:
- https://zoom.us/webinar/register/3117176913600/WN_Ev_1s7l2RUKmIQJNdko5iA本新聞稿包含《證券法》第27A條修正案和《證券交易法》第21E條修正案的“前瞻”聲明。這些聲明包括,但不限於,公司的有關臨床試驗的計劃。前瞻性聲明基於目前的預期和假設,並且可能會受到可能導致實際結果與預期結果不同的風險和不確定性的影響。此類風險和不確定性包括,但不限於,患者招募、搏君樂單抗的有效性、以及公司臨床試驗評估Buntanetap的療效、安全性和耐受性的時間、有效性和預期結果等方面的風險。額外的風險因素在公司向美國證券交易委員會的定期文件中有詳細描述,包括在公司的年度報告表10-K和季度報告表10-Q的“風險因素”部分中列出的風險因素。本新聞稿中的所有前瞻性聲明均基於公司在本發佈日期之前可獲得的信息。公司明確否認任何更新或修訂其前瞻性聲明的義務,無論是因爲新信息、未來事件還是其他原因,除非法律要求。
About Buntanetap: Buntanetap (formerly known as Posiphen or ANVS401) targets neurodegeneration by inhibiting the formation of multiple neurotoxic proteins, including amyloid beta, tau, alpha-synuclein, and TDP43. By improving synaptic transmission, axonal transport, and reducing neuroinflammation, Buntanetap aims to reverse neurodegeneration in AD, PD, and other neurodegenerative diseases.
關於Annovis Bio Inc.:總部位於賓夕法尼亞州的Malvern市,Annovis Bio公司專注於解決像AD和PD這樣的神經退行性疾病中的神經退化問題。該公司的創新方法針對多種神經毒性蛋白,旨在恢復大腦功能,並提高患者的生活質量。有關更多信息,請訪問Annovis Bio網站。
About Annovis Bio Inc.: Headquartered in Malvern, Pennsylvania, Annovis Bio Inc. is dedicated to addressing neurodegeneration in diseases such as AD and PD. The company's innovative approach targets multiple neurotoxic proteins, aiming to restore brain function and improve the quality of life for patients. For more information, visit www.annovisbio.com and follow us on LinkedIn and X.
Annovis Bio公司網站。www.annovisbio.com和我們一起LinkedIn和頁面。X.
Forward-Looking Statements
前瞻性聲明
This press release contains "forward-looking" statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements include, but are not limited to, the Company's plans related to clinical trials. Forward-looking statements are based on current expectations and assumptions and are subject to risks and uncertainties that could cause actual results to differ materially from those projected. Such risks and uncertainties include, but are not limited to, those related to patient enrollment, the effectiveness of Buntanetap, and the timing, effectiveness, and anticipated results of the Company's clinical trials evaluating the efficacy, safety, and tolerability of Buntanetap. Additional risk factors are detailed in the Company's periodic filings with the SEC, including those listed in the "Risk Factors" section of the Company's Annual Report on Form 10-K and Quarterly Reports on Form 10-Q. All forward-looking statements in this press release are based on information available to the Company as of the date of this release. The Company expressly disclaims any obligation to update or revise its forward-looking statements, whether as a result of new information, future events, or otherwise, except as required by law.
早期AD患者的有效性
Contacts
聯繫方式
Annovis Bio, Inc.
101 Lindenwood Drive
Suite 225
Malvern, PA 19355
www.annovisbio.com
Annovis Bio, Inc.
101 Lindenwood Drive
225套房
馬爾文,PA 19355
www.annovisbio.com
Investor Contact
投資者聯繫方式
Scott McGowan
InvestorBrandNetwork (IBN)
Phone: 310.299.1717
IR@annovisbio.com
Investor Website
Scott McGowan
InvestorBrandNetwork(IBN)
電話:310.299.1717
IR@annovisbio.com投資者網站
Attachments
附件
Efficacy in Early AD Patients
其先導藥物候選搏君樂單抗在APOE4攜帶者和非攜帶者中均展現出統計學顯著的療效和安全性
Buntanetap showed statistically significant efficacy and safety in both carriers and non-carriers of Apolipoprotein E4 (APOE4), a genetic cause of AD.
其先導藥物候選搏君樂單抗在APOE4攜帶者和非攜帶者中均展現出統計學顯著的療效和安全性,而APOE4是AD的基因原因之一。
Comparable Safety
相似的安全性
Buntanetap was found to be equally safe in both APOE4 carriers and non-carriers, with no instances of ARIA.
發現Buntanetap在APOE4攜帶者和非攜帶者中同樣安全,沒有ARIA發生。
Source: Annovis Bio, Inc.
消息來源:annovis bio,inc.
譯文內容由第三人軟體翻譯。