AxoGen (AXGN) Initiates BLA Submission for Avance Nerve Graft
AxoGen, Inc. AXGN announced the initiation of rolling submission of a Biologics License Application (BLA) seeking licensure for its human nerve allograft candidate, Avance Nerve Graft, to the FDA on May 15.
The initial submission includes the complete non-clinical data package. The company will submit the remaining Clinical and Chemistry, Manufacturing and Controls components in the upcoming months. The rolling submission is expected to be completed by the third quarter of 2024.
Following a potential approval, AxoGen’s Avance Nerve Graft will be available for bridging severed peripheral nerves without the comorbidities associated with a second surgical site.
Price Performance
In the past six months, AXGN shares have gained 1.5% compared with the industry’s rise of 24.9%. The S&P 500 has gained 17.5% in the same time frame.
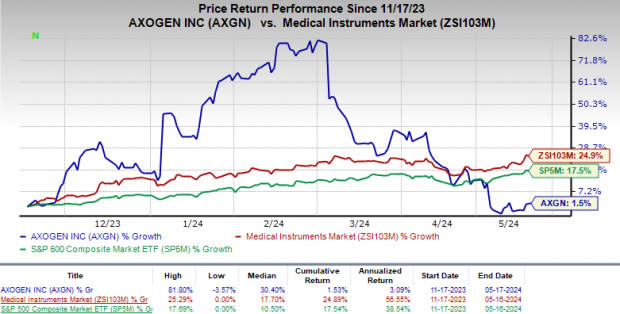
Image Source: Zacks Investment Research
More on the News
AxoGen’s Avance Nerve Graft was granted Regenerative Medicine Advanced Therapy designation by the FDA in 2018. This designation is given to therapies intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition and has the potential to address unmet medical needs. The designation will allow AxoGen to request for priority review following the completion of the rolling submission for the BLA. A priority review for the BLA will reduce the FDA review period from 10 months to six months, accelerating the approval process.
The company expects a potential approval for Avance Nerve Graft in mid-2025.
Other Potential Products
AxoGen strides toward providing innovative and clinically proven solutions to restore nerve function and quality of life for patients with peripheral nerve injuries.
The company’s other products include Axoguard Nerve Connector, a porcine (pig) submucosa extracellular matrix (ECM) coaptation used for tensionless repair of severed peripheral nerves.
Axoguard Nerve Protector, AxoGen’s porcine submucosa ECM product, is used for wrapping and protecting damaged peripheral nerves and reinforcing nerve reconstruction while preventing soft tissue attachments.
AxoGen’s Axoguard Nerve Cap, a porcine submucosa ECM product, is used to protect a peripheral nerve end and separate the nerve from the surrounding environment to reduce the development of symptomatic or painful neuroma.
The company’s Avive+ Soft Tissue Matrix, a multi-layer amniotic membrane allograft, is intended to protect and separate tissues in the surgical bed during the critical phase of tissue repair.
AxoGen’s Axotouch Two-Point Discriminator is used to measure the innervation density of any surface area of the skin.
Notable Developments
In April 2024, AxoGen announced the first surgical implantations of its newest product, Avive+ Soft Tissue Matrix. Avive+ Soft Tissue Matrix is a resorbable, multi-layer, placenta-based allograft that provides temporary protection and tissue separation during the critical phase of healing for nerves.
AxoGen, Inc. Price
AxoGen, Inc. price | AxoGen, Inc. Quote
Zacks Rank & Stocks to Consider
AXGN carries a Zacks Rank #3 (Hold) at present.
Some better-ranked stocks in the broader medical space that have announced quarterly results are Align Technology, Inc. ALGN, Ecolab ECL and Boston Scientific Corporation BSX.
Align Technology, carrying a Zacks Rank of 2 (Buy), reported first-quarter 2024 adjusted EPS of $2.14, beating the Zacks Consensus Estimate by 8.1%. Revenues of $997.4 million outpaced the consensus mark by 2.6%. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Align Technology has a long-term estimated growth rate of 6.9%. ALGN’s earnings surpassed estimates in three of the trailing four quarters and missed once, the average surprise being 5.9%.
Ecolab, carrying a Zacks Rank of 2 at present, has an estimated long-term growth rate of 13.3%. ECL’s earnings surpassed estimates in each of the trailing four quarters, with the average surprise being 1.7%.
Ecolab’s shares have rallied 33.8% against the industry’s 9.3% decline in the past year.
Boston Scientific reported first-quarter 2024 adjusted EPS of 56 cents, beating the Zacks Consensus Estimate by 9.8%. Revenues of $3.86 billion surpassed the Zacks Consensus Estimate by 4.9%. It currently carries a Zacks Rank #2.
Boston Scientific has a long-term estimated growth rate of 12.5%. BSX’s earnings surpassed estimates in all the trailing four quarters, the average surprise being 7.5%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Boston Scientific Corporation (BSX) : Free Stock Analysis Report
Ecolab Inc. (ECL) : Free Stock Analysis Report
Align Technology, Inc. (ALGN) : Free Stock Analysis Report
AxoGen, Inc. (AXGN) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 