Editor's note: On May 9, the Drug Evaluation Center (CDE) of the China National Drug Administration approved Laikai Pharmaceutical's LAE102 (ActRIa monoclonal Antibody) for clinical trials (IND) of new drugs to treat obese patients. So far, LAE102 has been approved by both the Chinese and US IND. Affected by this news, when the market opened the next day, Laikai Pharmaceutical's stock price once surged 22.29%, fully reflecting investors' optimistic expectations about LAE102's future commercialization potential. Such a positive market response not only brought capital-level support to Laikai Pharmaceutical, but also injected strong confidence into the company's future R&D and marketing activities. The company said it is speeding up preparations for the Phase I clinical trial and will soon start recruiting subjects for obesity.
Back in mid-2017, when Lu Xiangyang took young Dr. Zhang Ruipeng and started the first project, early research on ActRII related targets, at the Laikai Pharmaceutical Laboratory, he never anticipated that the diet drug track would take center stage in 2024. Lai Kai's lab is very small, and it's not at all comparable to when he was in a foreign company, but in the team's mind, his enthusiasm for entrepreneurship and scientific research was burning.
If we want to review and summarize Dr. Lu Xiangyang's nearly 20 years of research and development experience at multinational pharmaceutical companies, two points are worth mentioning: one is that he was awarded the Novartis Leading Scientist honorary award by Novartis VIVA in 2012; the other is that he is one of the co-inventors of the FDA's breakthrough therapy Bimagrumab (ActRII monoclonal antibody), which is currently used for potential fat loss and muscle preservation indications. Bimagrumab's company was acquired by Lilly last year for about 1.9 billion US dollars.
However, Lu Xiangyang resolutely decided to step out of the comfort zone of foreign companies and start his own business, because he always wanted to make a few real new molecules, new mechanisms, and new concept drugs in his own hands. Since the establishment of Lai Kai Pharmaceutical, on the one hand, he has promoted the anti-tumor AKT inhibitors introduced from Novartis to phase III clinical trials — this is a guarantee of Lai Kai's recent revenue and profit; on the other hand, Lai Kai's early team has never stopped the pace of independent discovery.
On May 9, Laikai Pharmaceutical recently announced that its self-developed LAE102 (ActRIIa monoclonal antibody) has received IND approval from CDE to treat obese patients. Some people said it was a “hot spot,” and Lu Xiangyang and his team laughed softly — after 7 years of accumulation, they finally made a lot of progress.
1. Current status of diet pills: weight loss? That's not enough! Building muscle and losing fat is the future
Let's first take a look at a set of data. According to the World Health Organization, the number of obese people in the world has exceeded 1 billion in 2022, accounting for about one-eighth of the world's total population. Of these, about 159 million obese people are children or adolescents between the ages of 5 and 19, and 889 million are adults.
Francesco Blanca, director of WHO's Department of Nutrition and Food Safety, further stated, “Obesity is a global problem nowadays.” Morgan Stanley estimates that the global obesity drug market is expected to exceed US$54 billion by 2030.
The global obese population continues to rise, and factors such as the explosion of the “magic drug for weight loss” simeglutide (GLP-1) and Jia Ling's “hot and hot” weight loss popularity have directly driven the rapid development of the weight loss products market. The stock prices of Eli Lilly and Novo Nordisk continued to hit new highs.
However, although GLP-1, known as the “magic drug for weight loss,” leads the weight loss drug market, studies have shown that nearly 40% of the weight lost by participants with this type of drug is muscle, and muscle loss increases the risk of cardiovascular diseases, osteoporosis, etc.
So just losing fat isn't enough. Value as a supporting target for GLP-1's “next level” gradually emerged, and ActRII, an emerging target, came to prominence.
Unlike GLP-1 drugs that simply lose weight, ActRII monoclonal antibodies can reduce fat accumulation while promoting muscle gain by blocking the ActRII signal. “Two birds with one stone” directly attacks pain points, which undoubtedly brings new room for imagination to the market.
2. Competitive landscape: the world's only potential drug for ActRII obesity indications under development
The Activin pathway is important in obesity, muscle atrophy, pulmonary hypertension, and oncology. As an activin receptor expressed in both adipose and muscle cells, ActRII (activin type II receptor/ activin type II receptor, including ActRII/ ActRIIb) activates its signals, causing muscle atrophy and accumulation of fat.
In other words, blocking this pathway can be expected to increase the body's muscle mass while reducing fat accumulation.
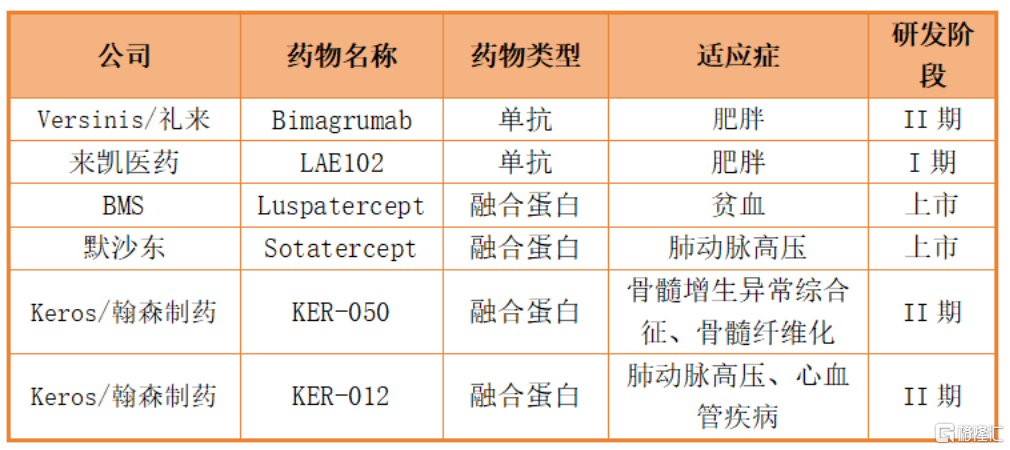
Judging from the competitive pattern of ActRII targets around the world, although many drugs have been developed for this target, most drugs under development mainly target indications such as blood diseases, pulmonary hypertension, and cancer, and mostly exist in the form of ActRIIA/b fusion proteins. In this field, there are relatively few ActRII antibody drugs that target obesity indications and have entered the clinical research stage. Currently, there are only two drugs, Bimagrumab, purchased by Lilly at a sky-high price, and LAE102 independently developed by Laikai Pharmaceuticals.
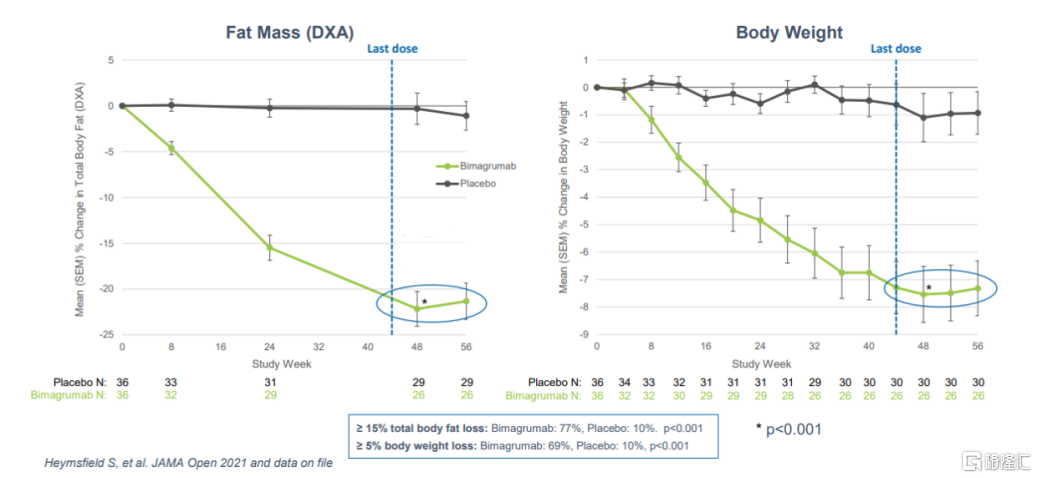
Bimagrumab has initially verified its effects on muscle gain and fat loss. According to the results of a phase II study of Bimagrumab in treating patients with type 2 diabetes and obesity or overweight in the authoritative medical journal JAMA Network Open, compared with placebo, Bimagrumab can reduce patients' fat mass by about 20.5% and increase fat-free body weight by 3.6%.
Furthermore, judging by the frequency of administration every four weeks, the 44th week was the last dose of Bimagrumab, so the 48th week results were the main endpoint data. As can be seen from the image below, unlike many treatments based on intestinal insulin (incretin), patients did not observe weight gain within 8 weeks of discontinuing treatment. To date, safety data for Bimagrumab show that side effects include muscle cramps and diarrhea, which occur early in treatment and are mostly mild.
Source: Public Information
At the same time, the phase 2b clinical study of Bimagrumab combined with simeglutide to treat obesity has also completed patient enrollment. The top-line data is expected to be read out in the second half of 2024.
Source: Public Information
Unlike Bimagrumab, Lai Kai's LAE102 is a selective monoclonal antibody targeting ActriIa. It specifically targets ActRIIa targets and plays a critical role in muscle regeneration and lipid metabolism.
Positive results from preclinical studies further confirm LAE102's potential: when LAE102 is used in combination with GLP-1 receptor agonists such as simeglutide, it can not only further reduce fat, but also significantly reduce muscle loss that may be caused by GLP-1 receptor agonists.
According to Lai Kai's latest announcement and 2023 report, LAE102 has obtained IND approval from China and the US for obesity indications. The company has made thorough preparations and will begin LAE102 clinical research as soon as possible. It can be judged that if subsequent LAE102 clinical trials go well and achieve positive results, it can be expected to become a breakthrough drug for the treatment of obesity. At the same time, it also once again certifies Laikai Pharmaceutical's innovative ability and R&D strength in the biomedical field.
3. Next step: Lai Kai's follow-up products for muscle gain and fat loss and anti-aging continue
Further, LAE102 is a potential global first ActriIa monoclonal antibody, and its development significance is not limited to obesity treatment — LAE102's multiple efficacy makes it potentially useful in various fields such as muscle regeneration, immune activation, hematopoietic development, and tumor growth control.
In addition, there are at least two antibodies for muscle regeneration and other disease indications in LAE102's successor product line — LAE123, an ActriIb selective antibody, and LAE123, an ActriIb dual-target inhibitor. According to the company's 2023 annual report, the Lai Kai team has accumulated rich experience and deep expertise in this specific field, and is developing more drug candidates to maximize the value of targeting ActRII receptors.
Aging is a natural law that is hard for humans to resist. One of the major symptoms is loss of muscle mass — muscle tissue deteriorates physiologically as we age, mainly due to loss of muscle mass, which is medically known as muscle atrophy.
Quoting a study published by the Beijing Municipal Health Commission, adults gradually lose muscle mass (including a gradual decline in muscle strength) starting at age 40, especially after age 75, and may lose 15% of muscle mass every 10 years. Among the elderly aged 60 to 70, the prevalence of sarcopenia is between 5% and 13%, while among the elderly aged 80 and above, the prevalence rate soared to 11% to 50%.
China's population is rapidly aging, and a drug target that promotes muscle regeneration may help address these concerns and problems. In the author's opinion, this indicates that LAE102 and subsequent products are expected to become a revolutionary drug with both muscle gain and fat loss and anti-aging functions in the field of innovative drugs in the future. The future room for imagination should not be underestimated.
4. Summary
Overall, in the next weight loss race of INcretins such as GLP-1RA, muscle-building therapy began to take the lead, and Laikai Pharmaceutical, which is the world's first LAE102, has shown its unique advantages and growth potential.
From an investment perspective, as Laikai Pharmaceutical continues to advance the clinical progress of LAE102 and other drugs under development, it is believed that subsequent companies will continue to bring more surprises in the development of innovative drugs, and their positive market performance and performance growth will eventually be reflected in the capital market.
