Ironwood (IRWD) Q1 Earnings & Revenues Fall Shy of Estimates
Ironwood Pharmaceuticals, Inc. IRWD reported an adjusted loss of 2 cents per share for first-quarter 2024 in contrast to the Zacks Consensus Estimate of earnings of 18 cents per share. The company had recorded adjusted earnings of 25 cents per share in the year-ago quarter.
Total revenues in the reported quarter were $74.9 million, which significantly missed the Zacks Consensus Estimate of $103 million. The top line decreased almost 28% year over year due to a $30.0 million gross-to-net change in estimate for the sole marketed drug, Linzess (linaclotide), recorded to collaborative arrangements revenues.
Quarter in Detail
As reported by partner AbbVie Inc. ABBV, Ironwood’s marketed product — Linzess — generated net sales of $256.6 million in the United States, up 3% year over year, driven by strong demand. Total prescriptions for Linzess increased 10% year over year.
Ironwood and AbbVie equally share Linzess’ brand collaboration profits and losses. IRWD’s share of net profit from the sales of Linzess in the United States (included in collaborative revenues) totaled $71.7 million, declining almost 29.4% year over year. Linzess share of profit was affected by the $30.0 million reduction, discussed above.
Linzess’ collaborative revenues from U.S. sales missed our model estimate of $100.3 million.
ABBV received FDA approval for the expanded use of Linzess for treating functional constipation in pediatric patients aged 6-17 years in June 2023. Following this nod, Linzess became the first and only FDA-approved prescription therapy for functional constipation in pediatric patients.
Ironwood has agreements with two partners — Astellas Pharma and AstraZeneca AZN — related to the development and commercialization of Linzess in Japan and China, respectively.
Both Astellas and AstraZeneca have exclusive rights to develop and market the drug in their respective territories. Astellas and AZN are liable to pay royalties to Ironwood on net Linzess revenues earned in their regions.
Ironwood recorded $3.2 million in royalties and other revenues, up 28% from the prior-year quarter’s figure.
Operating expenses (including research and development expenses, selling, general and administrative expenses and restructuring expenses) in the reported quarter were $63.9 million, up 45.2% year over year.
As of Mar 31, 2024, Ironwood had cash and cash equivalents worth $121.5 million compared with $92.2 million as of Dec 31, 2023.
2024 Guidance
Owing to a gross-to-net change in estimate for Linzess sales, IRWD lowered its revenue guidance for 2024.
The company now expects total revenues in the range of $405-$425 million for 2024 compared with the earlier projection of $435-$455 million. The company now anticipates U.S. sales of Linzess to decline in the mid-single digit range in 2024. Previously, U.S. sales of Linzess were expected to grow in low-single digits.
In response to the lowered revenue guidance as well as sales expectations for Linzess, the stock declined 17% on May 9.
Shares of Ironwood have plunged 41.1% year to date compared with the industry’s decline of 7.9%.
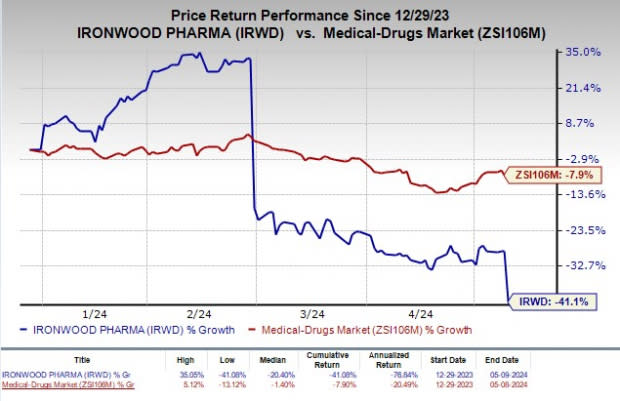
Image Source: Zacks Investment Research
Meanwhile, the company now expects to deliver adjusted EBITDA of more than $120 million for 2024 versus the earlier projection of more than $150 million.
Pipeline Updates
With the acquisition of VectivBio in June 2023, Ironwood acquired the rights to develop and commercialize apraglutide.
Ironwood is evaluating the safety and efficacy of once-weekly subcutaneous apraglutide in the pivotal phase III STARS study for reducing parenteral support (PS) dependency in adult patients with short bowel syndrome with intestinal failure (SBS-IF). In February 2024, the company announced positive top-line data from the STARS study, which demonstrated relative change from baseline in actual weekly PS volume, comparing apraglutide versus placebo at week 24 of treatment – the primary endpoint of the study.
Based on positive data from the STARS study, Ironwood plans to submit a new drug application for apraglutide in SBS-IF at the earliest.
Apraglutide is also being evaluated in a phase II exploratory study, STARGAZE, for patients with steroid-refractory gastrointestinal acute Graft-versus-Host Disease (SR GI aGVHD). In March 2024, the company announced positive primary data for up to day 91 from the STARGAZE study. Data from the study for up to 91 days showed treatment with apraglutide was well-tolerated with an acceptable safety profile. The STARGAZE study will continue with its two-year endpoint, wherein apraglutide will be re-investigated for safety and efficacy.
Ironwood is developing two other pipeline candidates, IW-3300 and CNP-104.
A phase II proof-of-concept study is evaluating IW-3300 for the potential treatment of visceral pain conditions, such as interstitial cystitis/bladder pain syndrome and endometriosis.
Meanwhile, Ironwood, in collaboration with COUR Pharmaceuticals, is developing CNP-104 for treating primary biliary cholangitis (PBC). A clinical study is being conducted by COUR Pharmaceuticals to evaluate the safety, tolerability, pharmacodynamic effects and efficacy of CNP-104 in PBC patients. Top-line data from the same is expected in the third quarter of 2024.
Ironwood Pharmaceuticals, Inc. Price, Consensus and EPS Surprise
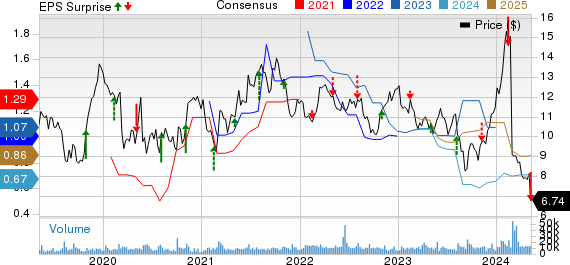
Ironwood Pharmaceuticals, Inc. price-consensus-eps-surprise-chart | Ironwood Pharmaceuticals, Inc. Quote
Zacks Rank & Stock to Consider
Ironwood currently carries a Zacks Rank #3 (Hold).
A top-ranked stock in the healthcare sector is Entera Bio Ltd. ENTX, sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Entera Bio’s 2024 loss per share have narrowed from 75 cents to 25 cents. Year to date, shares of ENTX have surged 308.3%.
ENTX’s earnings beat estimates in three of the trailing four quarters and missed the same once, the average surprise being 10.66%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Ironwood Pharmaceuticals, Inc. (IRWD) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
Entera Bio Ltd. (ENTX) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 