Global Healthcare CRO Services Market Report 2024-2028 & 2033 Featuring Profiles of Laboratory Corporation of America, IQVIA, Icon, PPD, Wuxi Apptec Co

Global Healthcare CRO Services Market
Dublin, March 26, 2024 (GLOBE NEWSWIRE) -- The "Healthcare CRO Services Global Market Report 2024" report has been added to ResearchAndMarkets.com's offering.
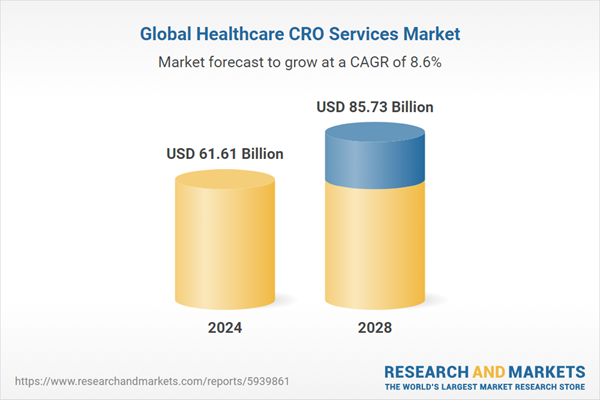
The healthcare CRO services market has grown strongly in recent years. It will grow from $56.8 billion in 2023 to $61.61 billion in 2024 at a compound annual growth rate (CAGR) of 8.5%. The growth witnessed in the historical period can be ascribed to the heightened emphasis on regulatory and medical affairs services provided by CROs, the globalization of clinical trials, the escalating complexity of clinical trials, the digital transformation in clinical trials, and the rising prevalence of chronic diseases.

The market is expected to see strong growth in the next few years. It will grow to $85.73 billion in 2028 at a compound annual growth rate (CAGR) of 8.6%. The anticipated growth in the forecast period can be attributed to the ascent of biologics and cell and gene therapies, a heightened focus on rare diseases, emphasis on cost-effectiveness and speed, an increasing demand for real-world data, a focus on patient-centricity, and the growth of the biopharmaceutical pipeline. Major trends in the forecast period include the integration of artificial intelligence (AI), incorporation of machine learning (ML), considerations of environmental, social, and governance (ESG) factors, and the escalation of research and development (R&D) investments by pharmaceutical companies.
The anticipated growth in the healthcare CRO services market is expected to be propelled by the increase in healthcare expenditures. For instance, as of February 2023, according to the Centers for Medicare and Medicaid Services, a federal agency in the United States, the country spent $4.3 trillion in 2021, equivalent to $12,914 per person, on healthcare. This reflected a 2.7% increase from the previous year, and healthcare spending accounted for 18.3% of the nation's GDP. Therefore, the upsurge in healthcare expenditures is a driving force behind the growth of the healthcare CRO services market.
The increasing number of clinical trials is expected to further boost the growth of the healthcare CRO services market. For instance, according to a report from Xtalks, a Canada-based web news and information network, there were 452,604 clinical studies registered on ClinicalTrials.gov as of May 2023, representing a significant increase from the over 365,000 registered trials recorded in early 2021. Therefore, the growth in clinical trials is contributing to the expansion of the healthcare CRO services market.
Major players in the healthcare CRO services market are adopting a strategic partnership approach to provide advanced Proteomics CRO services to biopharmaceutical and biomarker clients on a global scale. Strategic partnerships involve companies leveraging each other's strengths and resources for mutual benefits and success. In January 2023, Bruker Corporation, a US-based manufacturer of scientific instruments, formed a partnership with Biognosys AG, a Switzerland-based healthcare CRO services provider. The collaboration between Bruker and Biognosys is expected to create unique synergies, combining Biognosys' extensive range of proprietary proteomics services, software, and kits with Bruker's innovative timsTOF platform. This strategic partnership will result in the establishment of Biognosys' inaugural laboratory for advanced proteomics CRO services in the United States.
Major companies in the healthcare CRO services market are also innovating by developing products such as clinical trial monitoring solutions to expand their customer bases, boost sales, and increase revenue. A clinical trial monitoring solution is a comprehensive system or set of tools designed to oversee and manage various aspects of clinical trials. For example, in April 2022, Tata Consultancy Services (TCS), an India-based information technology (IT) services and consulting company, introduced a Risk-Based Monitoring solution. This innovative solution utilizes advanced statistical algorithms to proactively identify and address study and site risks in biopharmaceutical and Contract Research Organizations (CROs). Using data science, the solution accurately predicts outcomes related to site workload and risks, enabling stakeholders to implement informed monitoring strategies. The Risk-Based Monitoring solution provides unprecedented insights into missing data, enhancing data quality, consistency, and early detection of trends. With the potential for up to a 30% efficiency gain in site monitoring, the solution's automation streamlines processes, reduces site workload, ensures compliance, and accelerates product speed-to-market.
In July 2021, ICON PLC, an Ireland-based clinical research organization, completed the acquisition of PRA Health Sciences for $12 billion. Through this acquisition, ICON plc aimed to enhance operations to transform clinical trials and expedite the commercial success of biopharma clients in developing essential pharmaceuticals and medical devices. Additionally, the acquisition allowed ICON plc to focus on leveraging data, utilizing technology, and reaching diverse patient populations to expedite the development of new drugs. PRA Health Sciences Inc. is a US-based contract research organization (CRO) that offers data solutions and outsourced clinical development services.
Report Scope
Markets Covered:
1) By Service Type: Early Phase Development; Clinical Development Services; Laboratory Services; Consulting Services
2) By Therapeutic Area: Oncology; CNS Disorder; Cardiovascular Disease; Metabolic Disease; Infectious Disease; Diabetes; Other Therapeutic Areas
3) By End-user: Pharmaceutical Companies; Medical Device Companies; Academic Institutes
Key Companies Mentioned: Laboratory Corporation of America Holdings.; IQVIA Inc.; Icon Plc.; PPD Inc.; Wuxi Apptec Co. Ltd.
Time Series: Five years historic and ten years forecast
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments
Key Attributes
Report Attribute | Details |
No. of Pages | 175 |
Forecast Period | 2024 - 2028 |
Estimated Market Value (USD) in 2024 | $61.61 Billion |
Forecasted Market Value (USD) by 2028 | $85.73 Billion |
Compound Annual Growth Rate | 8.6% |
Regions Covered | Global |
A selection of companies mentioned in this report includes:
Laboratory Corporation of America Holdings.
IQVIA Inc.
Icon PLC
PPD Inc.
Wuxi Apptec Co. Ltd.
Syneos Health Inc.
Parexel International Corporation
Charles River Laboratories International Inc.
Covance Inc.
Medpace Inc.
Pharmaron Beijing Co. Ltd.
BioClinica Inc.
Chiltern International Ltd.
Evotec SE
PSI CRO AG
Premier Research Group Ltd.
Worldwide Clinical Trials Inc.
Caidya
BioTelemetry Inc.
Syngene International Limited
Novotech CRO
Celerion
GVK Biosciences Private Limited
Synteract Inc.
Veristat LLC.
ACM Global Laboratories
ClinTec Luxembourg SA
Clinlogix LLC
For more information about this report visit https://www.researchandmarkets.com/r/abwzei
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
CONTACT: CONTACT: ResearchAndMarkets.com Laura Wood,Senior Press Manager press@researchandmarkets.com For E.S.T Office Hours Call 1-917-300-0470 For U.S./ CAN Toll Free Call 1-800-526-8630 For GMT Office Hours Call +353-1-416-8900


 Yahoo Finance
Yahoo Finance