Gacos Pharmaceuticals (1167.HK) presented data on the indications of the self-developed KRAS G12C inhibitor for pancreatic cancer and other solid tumors in the form of an oral report at the ASCO GI annual meeting in the US. Among pancreatic cancer indications, the objective remission rate is twice that of existing standard treatments, and the safety is better. With its excellent data, Tianfeng Securities gave Gacos a buying rating and gave a target price of HK$7.68, which has room to double the current price.
Pancreatic cancer is highly malignant, with a 5-year survival rate of 1%-5%. Currently, the main treatment is chemotherapy. In second-line pancreatic cancer patients and above, the standard treatment is irinotecan hydrochloride liposome combined with fluorouracil (5-FU) /folic acid (LV). The ORR is about 10%, and side effects above grade 3 are over 50%. Gacos's Golerese confirmed an objective remission rate of 41.9%, and side effects above grade 3 were only half of those of standard treatment.
The objective remission rates of Amgen's sotolacib and Mirati's adaglasib for pancreatic cancer were 21% and 33.3%, respectively. The efficacy data of golerese was significantly higher than that of similar products.
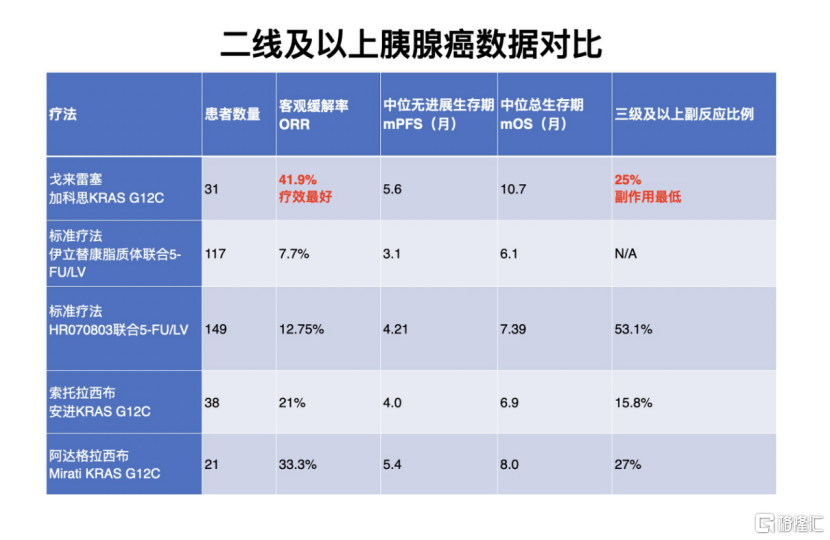
Golerese is the only product of its kind in the world to conduct registered clinical trials for pancreatic cancer, and has been certified as a breakthrough treatment in China. According to information, Gacos is currently actively communicating with the FDA to carry out registered clinical trials for pancreatic cancer in the US. Pacific Securities pointed out that Golaiser's pancreatic cancer indications are expected to go overseas.
The three major indications of Golerese layout and SHP2 in combination with the “sensitizer” SHP2 are expected to impact the frontline
In addition to pancreatic cancer, Golerese is also exploring other indications. Golerese's colorectal cancer indications are currently the most advanced in China. The single drug ORR is 62.8%, which is superior to overseas competitors.
There are about 40,000 new KRAS G12C mutant non-small cell lung cancer patients in China every year. Currently, no targeted drugs are available. The registration clinical trial of Golerese for non-small cell lung cancer was completed in 2023. It is expected that a new single-agent second-line treatment for non-small cell lung cancer will be submitted in the first half of 2024.
The more important application scenario for Golerese is to become a first-line therapy, and the key is to use it in combination with the “sensitizer” SHP2. Gacos is the second company in the world to promote SHP2 into clinical trials, and it is also the only company in the world other than Novartis that has these two major projects at the same time. The second-generation SHP2 inhibitor JAB-3312, known as the “global dark horse,” has stronger anti-tumor activity, lower dosage, and higher safety. According to data published by Gacos at the ESMO 2023 conference, the objective response rate was 86.7% (13/15) in the dose group using Golerese 800 mg and 2 mg JAB-3312. Currently, the objective mitigation rate of KRAS G12C, which has been marketed worldwide, is about 40%. Under the sensitizing effect of SHP2, the objective mitigation rate of KRAS G12C has doubled. This combination therapy is expected to surpass immunotherapy combined with chemotherapy and become a first-line treatment.
Although the median progression-free survival (mPFs) of combination therapy is not mature, the MPFs of golerese monotherapy were 9.6 months in people with second-line non-small cell lung cancer, and MPFs with combination therapy are expected to increase further based on this figure. Golerese and SHP2 are expected to replace chemotherapy combined with PD-1. Patients do not need to go to the hospital to inject the drug and can take the targeted drug at home. Furthermore, if Goleisse is approved for listing in the three major indications of non-small cell lung cancer, colorectal cancer, and pancreatic cancer, according to the plan, Tianfeng Securities predicts that the sales volume of this product in the Chinese market alone will exceed 1.5 billion yuan in 2028.
In terms of overseas markets for KRAS G12C inhibitors, the FDA's full approval of sotoracib was delayed. The conditional marketing of adaglasib for non-small cell lung cancer in Europe went through two iterations. Although it was approved for marketing in January 2024, its safety issues were not recognized by clinicians. Gacos may have regained global opportunities with the world's only registered clinical trial for pancreatic cancer. The excellent clinical data on pancreatic cancer and other solid tumors announced by ASCO GI this time became a strong impetus. Based on the incidence rate estimates by China Post Securities, the market potential of KRAS G12C in the US is 19 billion yuan.
The catalyst is dense and flexible, and Tianfeng Securities gave it a target price of HK$7.68
In addition to KRAS inhibitors and SHP2 inhibitors, Gacos has a differentiated pipeline layout with the world's top three potential, KRASMultiThree products, the inhibitor JAB-23425, the BET inhibitor JAB-8263, the Aurora A inhibitor JAB-2485, and the CD73 monoclonal antibody JAB-BX102, have entered the clinical phase; the three products of GUE inhibitor JAB-24114, LIF monoclonal antibody JAB-BX300, and PARP7 inhibitor JAB-26766 were approved for IND in 2023.
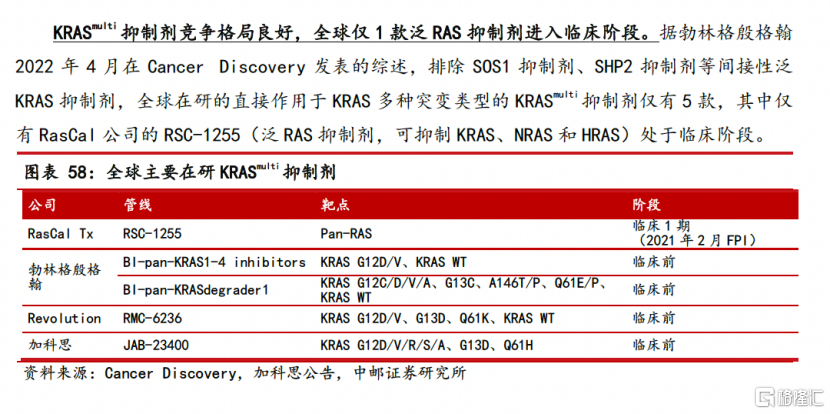
KRASMultiThe inhibitor JAB-23425 is an ultra-broad-spectrum targeted drug. Currently, only one pan-RAS project in the world is in the clinical phase. The world adds 2.7 million new patients with KRAS-related mutations every year, and China adds nearly 600,000 new patients with KRAS every year MultiThe mutant types that can be covered (G12D, G12V, G12R, G12S, G12A, G13D, G61H, etc.), the market potential is 8-10 times that of KRAS G12C. This project is expected to be submitted to IND in the first half of 2024. After the IND is approved, non-small cell lung cancer can be enrolled simultaneously through a basket clinical trial (basket clinical trial) (25% with KRAS)multi-mutation), colorectal cancer (35% with KRASMultimutation), pancreatic cancer (90% with KRASMultipatients with mutations), etc.
Gacos's BET inhibitor is a product that is underestimated in the market and has a billion dollar molecular potential. Recently, phase III clinical data of the BET inhibitor pelabrisib released by the German company Morphosys showed good treatment effects of BET inhibitors in medium- and high-risk bone marrow fibrosis. The stock price rose 45% on the day the data was released. Judging from public data, JAB-8263 is the most active BET inhibitor in similar clinical programs. It is expected to effectively inhibit the growth of various tumors at extremely low doses, and has a huge differentiating advantage over MorphoSys products.
Judging from overseas data from BET competitors, the combined use of BET and JAK is expected to replace JAK as a single drug as a first-line treatment plan for myelofibrosis. Currently, 1 billion US dollars in global sales of JAK inhibitors come from myelofibrosis indications.
Another underrated Gacos project is the self-developed oral P53 agonist JAB-30300, which is scheduled to be submitted to IND in the first half of this year. The P53 gene is one of the most common tumor suppressor genes in humans, and more than 50% of malignant tumors will mutate this gene. JAB-30300 is a highly selective oral activator designed for the P53 Y220C inactivation mutation. It achieves cancer suppression effects by restoring the normal function of P53. The current pioneer of the P53 target is the US company PMV. Early data showed an ORR of 23% (5/21), and the FDA has approved it to conduct single-arm phase II clinical trials.
In addition to targeted drugs, Gacos is also studying the next generation tumor immunotherapy, iAdc, to replace toxin loading with STING, an immunostimulant. Gacos previously revealed in its annual report that tumors completely disappeared after the first administration of JAB-X1800 (CD73 STING iAdc) in animal models. If this effect can be partially reproduced in humans, it will be a major advance in the field of tumor immunity. The American company Mersana's iADC product XMT-2056 was authorized to GSK at a total price of US$1.46 billion, which also fixed the BD transaction price for Gacos's iADC project.
Combined with the world's top three ongoing research pipelines and the advance commercialization layout of Golerese, Tianfeng Securities called Gacos a “dark horse for small molecule innovative drugs that break through unformable targets,” and expects the company's market capitalization to be HK$6.092 billion in 2024, with a target price of HK$7.68, which has room to double the current market value.
