①目前國內已有多個廠家的流感疫苗獲批上市,其中2022~2023年度流感季已有11家廠家供應; ②中泰證券研報指出,伴隨流感季的到來,建議關注相關檢測、疫苗及藥物公司投資機會。
財聯社12月2日訊(編輯 魏齊)中國疾控中心病毒病所國家流感中心主任王大燕表示,當前,正值流感的高發季節,對之前沒有接種流感疫苗的人群,接種流感疫苗仍然是有效的,建議大家儘早接種流感疫苗。在這裏,我特別指出,6個月以上的人群都可以接種流感疫苗,尤其是對於兒童及老年人、慢性病患者等高風險人群,在感染流感後發生重症的風險較高,接種流感疫苗可以有效降低發展爲重症和死亡的風險,希望大家積極接種。
11月23日,根據中國國家流感中心的數據,2023年第46周,南方省份哨點醫院報告的ILI%爲6.4%,高於前一週水平(5.5%),高於2020~2022年同期水平(3.7%、3.4%和3.0%),北方省份哨點醫院報告的ILI%爲6.2%,高於前一週水平(5.0%),高於2020~2022年同期水平(2.5%、2.8%和2.1%)。
 中泰證券研報指出,南、北方省份流感病毒檢測陽性率持續上升,可能由2個原因造成:①過去2年的防疫措施如帶口罩等積累了大量的易感人群;②近期流行的毒株爲A(H3N2)亞型,其傳染性更強、感染率更高。
中泰證券研報指出,南、北方省份流感病毒檢測陽性率持續上升,可能由2個原因造成:①過去2年的防疫措施如帶口罩等積累了大量的易感人群;②近期流行的毒株爲A(H3N2)亞型,其傳染性更強、感染率更高。
據世界衛生組織估計,全球每年約29萬-65萬人死於流感相關的呼吸疾病。每年的10月至次年3月是流感等呼吸道傳染病的高發季,接種流感疫苗是預防流感最簡單有效的方法。
按照疫苗所含組份,流感疫苗包括三價和四價,三價疫苗組份含有A(H3N2)亞型、A(H1N1)pdm09亞型和B型毒株的一個系,四價疫苗組份含兩個A亞型和B型Victoria系、Yamagata系。
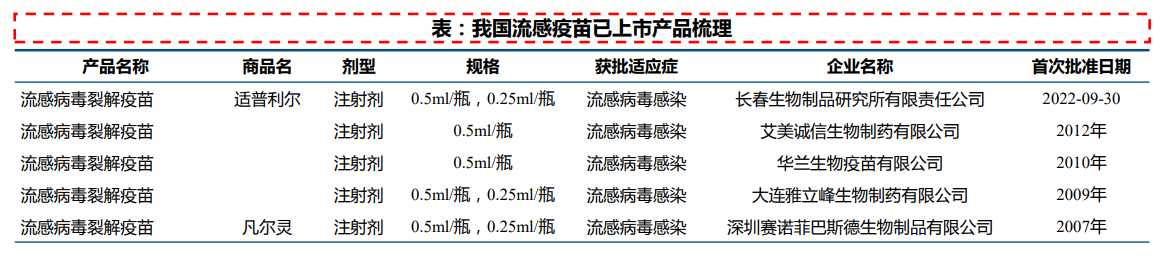
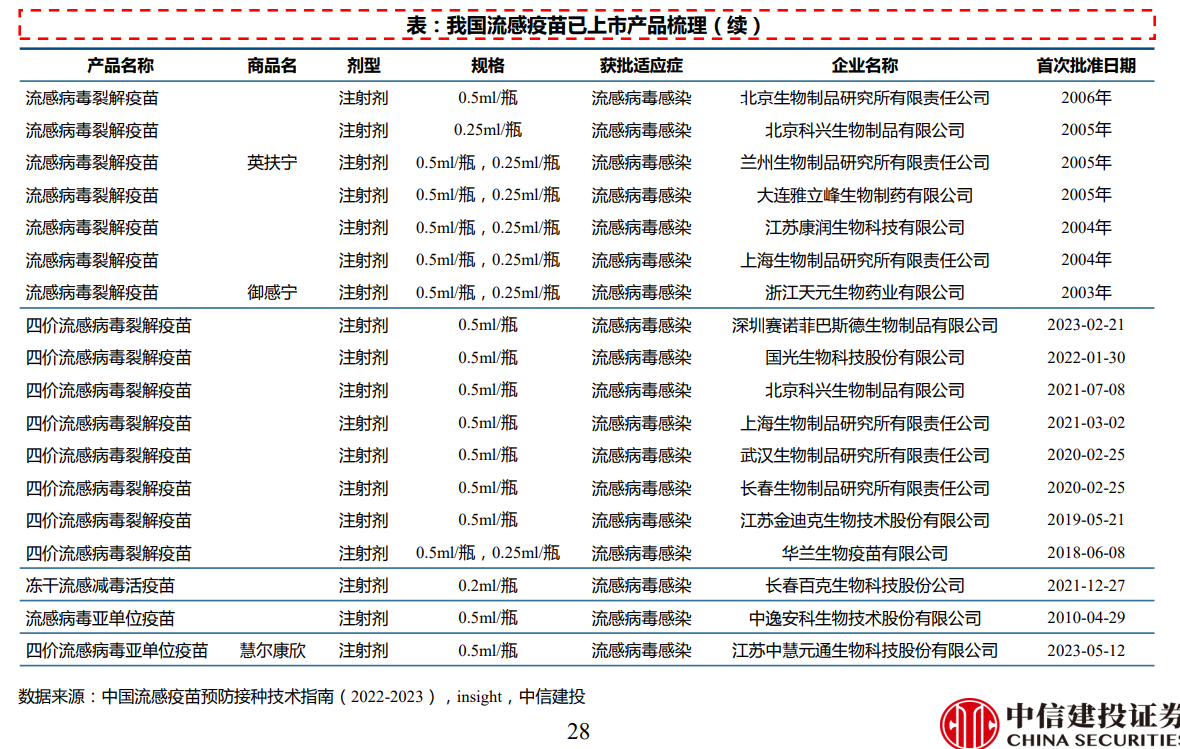
國內已批准上市的流感疫苗有三價滅活流感疫苗(IIV3)、四價滅活流感疫苗(IIV4)和三價減毒活疫苗(LAIV3)。目前國內已有多個廠家的流感疫苗獲批上市,其中2022~2023年度流感季已有11家廠家供應;另有多條管線處於臨床階段。
三價流感病毒裂解疫苗:目前已有12款疫苗上市,其中2022~2023年度流感季有5家廠家供應,包括長春所、科興生物、大連雅立峯、華蘭生物和賽諾菲巴斯德。
四價流感病毒裂解疫苗:目前已有8款疫苗上市,其中2022~2023年度流感季有7家廠家供應,包括長春所、科興生物、國光生技、華蘭生物、金迪克、上海所和武漢所。處於上市申報階段品種2個,來自智飛生物、大連雅立峯。處於臨床III期品種6個,除康泰生物、康潤生物和浙江天元外,另有智飛生物、中慧元通和科興生物的低年齡段管線。
流感病毒亞單位疫苗:目前三價和四價產品各有1款上市,三價爲中逸安科,四價爲中慧元通(2023年5月獲批上市)。處於臨床III期品種1個,爲中慧元通的低年齡段管線。處於臨床II期品種1個,爲長春所的低年齡段管線。
凍幹流感減毒活疫苗:目前有1款上市,來自百克生物。另有百克生物的液體劑型管線處於臨床II期。
市場份額方面,根據華經產業研究院數據,2022年全年共有459次流感疫苗批次,其中華蘭疫苗流感疫苗批簽發批次爲103批次,佔比22%,位居行業首位,其中四價流感成人劑型77批次,兒童劑型14批次,三價流感疫苗12批次。金迪克流感疫苗批簽發批次佔比約爲15%,排在第二位;此外,其他企業的佔比均不超過10%。
中泰證券研報認爲,流感不等於普通感冒,高發季節傳染危害不容忽視,伴隨流感季的到來,建議關注相關檢測、疫苗及藥物公司投資機會。①流感檢測:安圖生物、萬孚生物、復星診斷、邁克生物等;②流感疫苗:華蘭生物/華蘭疫苗、百克生物、金迪克、復星醫藥等;③流感藥物及產業鏈:南新制藥、東陽光藥、諾泰生物、博瑞醫藥、衆生藥業、上海醫藥、科倫藥業、石藥集團、雙鷺藥業、一品紅、萊茵生物等。

 中泰证券研报指出,南、北方省份流感病毒检测阳性率持续上升,可能由2个原因造成:①过去2年的防疫措施如带口罩等积累了大量的易感人群;②近期流行的毒株为A(H3N2)亚型,其传染性更强、感染率更高。
中泰证券研报指出,南、北方省份流感病毒检测阳性率持续上升,可能由2个原因造成:①过去2年的防疫措施如带口罩等积累了大量的易感人群;②近期流行的毒株为A(H3N2)亚型,其传染性更强、感染率更高。