伴隨診斷是通過測量人體內變異基因或蛋白的表達水平,瞭解不同患者對特定藥物的治療反應,篩選出最合適的用藥人羣並有針對性地進行個體化治療,從而改善其治療預後和降低保健開支。 20 年來, 國內外伴隨診斷產業發展迅猛。目前,行業處於初級階段,參與的企業大多是 Roche、 Abotte 等巨頭,格局相對分散,國內艾德生物等企業佈局較早,位於第一梯隊。
1、伴隨靶向治療實現個性化治療, 伴隨診斷助力精準醫療發展
廣證恆生指出,目前腫瘤藥物銷售前 10 的均為腫瘤靶向藥物, 佔 2017 年全球抗腫瘤處方藥市場 56.09%的份額, 預計 2022 年將達到 73%; 2017 年國內佔比 24.25%, 持續上升。
靶向治療是指在細胞分子水平上,針對細胞內的一個基因片段或一個蛋白分子的一種治療方法, 這些基因片段、蛋白分子既是致病位點,也是治病靶點。
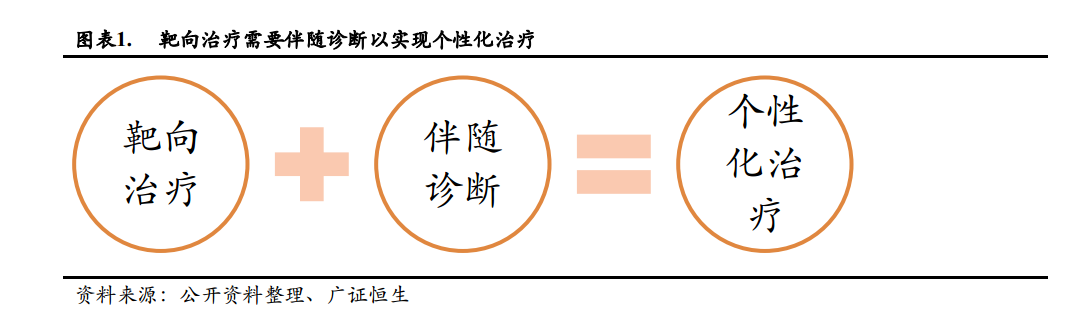
伴隨診斷服務於靶向治療, 在進行靶向治療之前,必須要對患者進行靶點檢測,只有「靶點陽性」的患者才能接受相應的靶向治療。 尤其是腫瘤領域的靶向治療,更需要伴隨診斷檢測靶點, 因為癌症的起因就是基因變異。
2、全球伴隨診斷市場以超過20%的增速達到30 億美元,國內龍頭初顯
廣證恆生指出,20年來,國內外伴隨診斷產業發展迅猛,FDA於2014年出臺兩個文件明文指導,美國已有40多種伴隨診斷產品獲批;國內伴隨診斷試劑仍按照體外診斷(IVD)試劑進行註冊管理,目前已有近200個國產基因檢測IVD產品獲批,臨牀上在腫瘤領域應用最多,佔比達70%以上。伴隨診斷產品應用最多的技術是熒光PCR,NGS技術代表着未來的發展方向。

據 MarketsandMarkets 數據,全球 2016 年伴隨診斷市場規模為 19 億美元,預計 2018 年將達到 31.3 億美元, 2016 年到 2022 年的年複合增長率將達到 22.78%,顯著高於全球 IVD 整體行業 6%的增長速度。作為體外診斷市場發展最快的細分領域之一,伴隨診斷在體外診斷市場的份額逐漸增大,伴隨診斷佔體外診斷市場佔比由 2010 年的 2.9%增長至 2021 年的 14.0%。

國內 2010 年伴隨診斷市場規模為 0.49 億美元,預計 2018 年將達到 3 億美元。 2010 年到 2021 年的年複合增長率為 28.0%, , 增速不僅高於全球水平,也顯著高於國內 IVD 行業約 15%的增速。
3、市場競爭格局:行業處於初級階段,玩家多是國際醫藥巨頭
全球伴隨診斷行業處於初級階段,參與的企業大多是 Roche、 Abotte 等巨頭,格局相對分散;國內眾多公司佈局有多款產品, 競爭激烈,艾德生物、燃石醫學等企業佈局較早,位於第一梯隊,專業的公開伴隨診斷公司估值上看, A 股上市公司艾德生物一直獨秀,百傲科技等新三板公司均集中在 5 億元左右。
4、伴隨診斷髮展趨勢:NGS 多基因檢測成為未來發展趨勢,液體活檢被廣泛看好
1)多種腫瘤生物標誌物聯合檢測可提高診斷的準確性,NGS本身具備高通量檢測的優勢以及能進行多基因聯合檢測的 PCR技術極具前景

2)液體活檢預計 5~15 年後市場將會走向成熟,屆時,全球液體活檢規模將達 230 億美元, 國內達375 億人民幣。


5、投資建議
廣證恆生建議關注擁有國內首款液體活檢產品、 NGS 腫瘤檢測產品進入特別審批通道的艾德生物。
廈門艾德生物醫藥科技股份有限公司由「千人計劃」國家特聘專家鄭立謀教授於 2008 年回國創辦,集腫瘤精準醫療診斷產品的研發、生產、銷售、服務為一體, 公司具備三類體外診斷產品生產、 經營資質及獨立臨牀醫學檢驗資質, 產品主要用於檢測腫瘤患者相關基因狀態,為腫瘤靶向藥物的選擇和個體化治療方案的制定提供科學依據。 短短的十年間已成為我國首家專業化的腫瘤精準醫療分子診斷產品研發生產企業,並於 2017 年在深圳創業板上市交易。

國內首款液體活檢產品獲批, NGS 腫瘤檢測產品「特別審批」。2018 年 1 月,艾德生物的基於 Super-ARMS 技術的人類 EGFR 突變基因檢測試劑盒獲批,是國內首個獲批的 EGFR 液體活檢試劑盒,也是目前唯一一款以奧西替尼伴隨診斷試劑標準獲批的產品,市場首發優勢明顯。
