MannKind Shares Are Falling Today: What's Going On?
MannKind Shares Are Falling Today: What's Going On?
MannKind Corporation (NASDAQ:MNKD) are trading lower Monday. The company released its six-month results from its Phase 3 INHALE-1 pediatric diabetes trial. Here's what you need to know.
曼恩凱德生物醫療公司(納斯達克:MNKD)週一股價下跌。該公司發佈了其第3期INHALE-1兒童糖尿病試驗的六個月結果。以下是你需要知道的。
What To Know: The trial investigated the use of Afrezza (insulin human) Inhalation Powder in children and adolescents aged 4 to 17 years. Its primary endpoint was a non-inferior change in HbA1c levels after 26 weeks of treatment.
重要信息:該試驗調查了在4至17歲的兒童和青少年中使用Afrezza(人用胰島素)吸入粉末。其主要終點是在26周治療後HbA1c水平的非劣效性變化。
The results showed that, while Afrezza met the non-inferiority criteria based on a modified intent-to-treat (mITT) analysis, a full intent-to-treat (ITT) analysis did not meet the predetermined non-inferiority margin, largely due to the variability introduced by one non-compliant patient. Despite this, the mITT analysis supported Afrezza's non-inferiority to multiple daily injections (MDI) of rapid-acting insulin.
結果顯示,雖然Afrezza在經過修訂的意向治療(mITT)分析中滿足非劣效性標準,但完全意向治療(ITT)分析並未達到預定的非劣效性界限,這在很大程度上是由於一名不合規患者所引入的可變性。儘管如此,mITT分析仍支持Afrezza在非劣效性方面與多次每日注射(MDI)快速作用胰島素的比較。
The study also showed no significant differences in lung function between the Afrezza and MDI groups, with both treatment groups maintaining stable FEV1 levels over the 26-week period. No major safety concerns, including hypoglycemia, were noted between the two treatment regimens.
研究還顯示,Afrezza組和MDI組之間在肺功能方面沒有顯著差異,兩個治療組在26週期間都保持了穩定的FEV1水平。在兩種治療方案之間沒有發現重大的安全性問題,包括低血糖。
Despite the encouraging efficacy and safety outcomes, the stock's decline reflects investor cautiousness, as the company now plans to meet with the U.S. Food and Drug Administration (FDA) in the first half of 2025 regarding a supplemental new drug application (sNDA) for Afrezza's use in pediatric populations. This meeting is crucial for the potential next steps in expanding the treatment's approval, which may contribute to further volatility in the stock price depending on the regulatory response.
儘管療效和安全性結果令人鼓舞,股票的下滑反映了投資者的謹慎,因爲該公司現在計劃在2025年上半年與美國食品和藥品監督管理局(FDA)召開會議,討論關於Afrezza在兒童人群中使用的補充新藥申請(sNDA)。這次會議對於擴大治療批准的潛在後續步驟至關重要,這可能導致股票價格的進一步波動,具體取決於監管反饋。
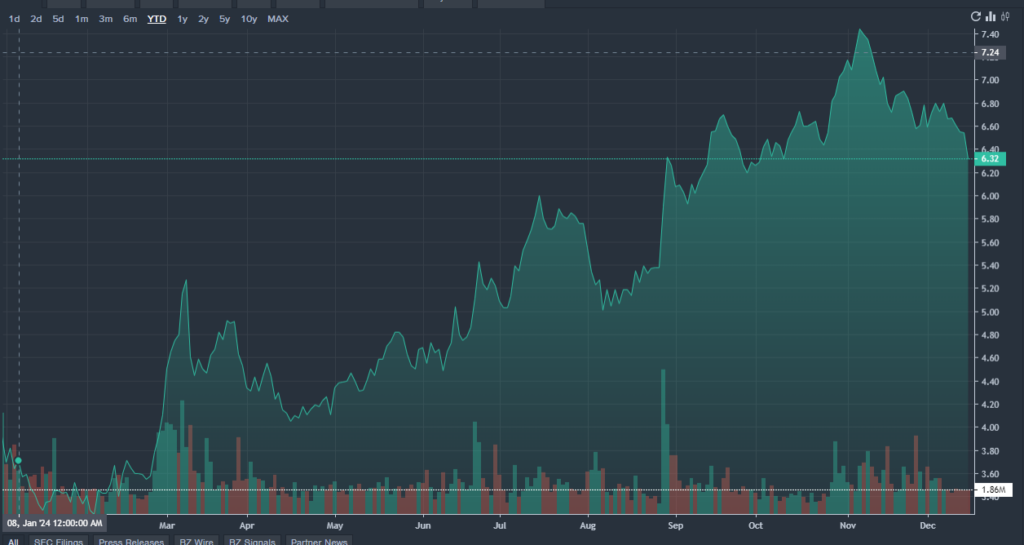
MNKD Price Action: MannKind shares were down 4.28% at $6.27 at the time of writing, according to Benzinga Pro.
MNKD價格動態:根據Benzinga Pro,曼恩凱德生物醫療的股票在撰寫時下跌了4.28%,報6.27美元。
- Nvidia And 5 Other Stocks Are Analyst's Top Semiconductor Picks For 2025, Sees 2 AI Trends
- 英偉達和其他5只股票是分析師2025年首選的半導體股票,看到2個人工智能趨勢
Image Via Shutterstock.
圖片來自Shutterstock。
譯文內容由第三人軟體翻譯。

 The study also showed no significant differences in lung function between the Afrezza and MDI groups, with both treatment groups maintaining stable FEV1 levels over the 26-week period. No major safety concerns, including hypoglycemia, were noted between the two treatment regimens.
The study also showed no significant differences in lung function between the Afrezza and MDI groups, with both treatment groups maintaining stable FEV1 levels over the 26-week period. No major safety concerns, including hypoglycemia, were noted between the two treatment regimens.