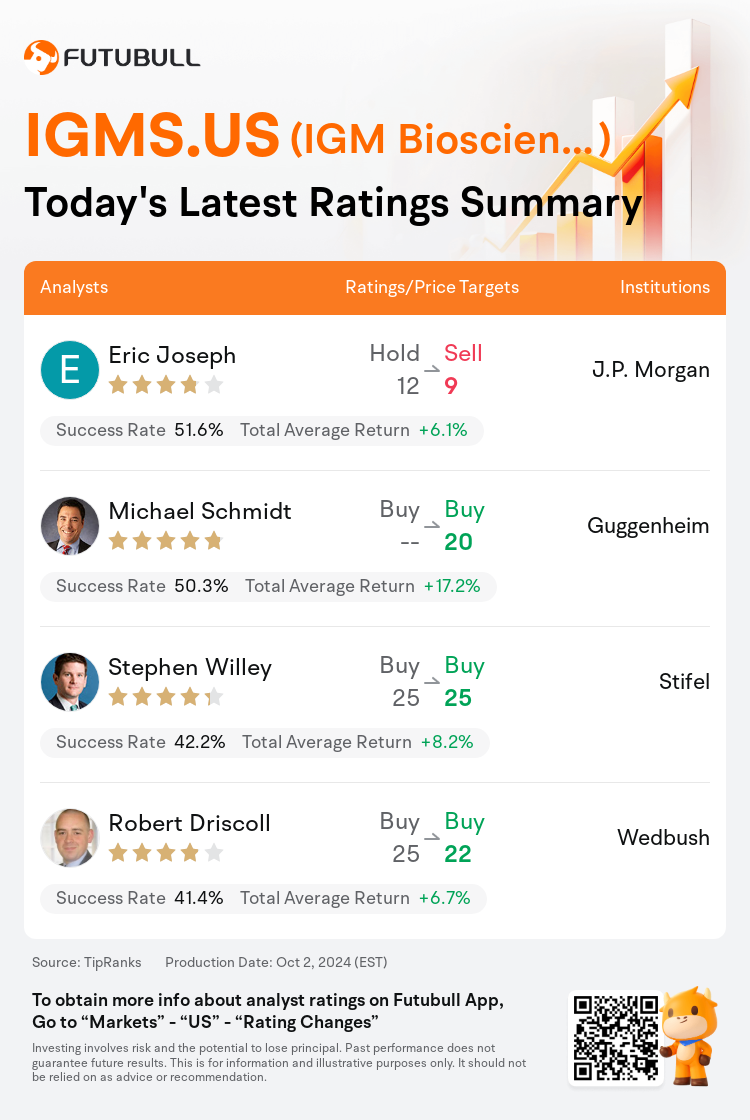
On Oct 02, major Wall Street analysts update their ratings for $IGM Biosciences (IGMS.US)$, with price targets ranging from $9 to $25.
J.P. Morgan analyst Eric Joseph downgrades to a sell rating, and adjusts the target price from $12 to $9.
Guggenheim analyst Michael Schmidt maintains with a buy rating, and sets the target price at $20.
 Stifel analyst Stephen Willey maintains with a buy rating, and maintains the target price at $25.
Stifel analyst Stephen Willey maintains with a buy rating, and maintains the target price at $25.
Wedbush analyst Robert Driscoll maintains with a buy rating, and adjusts the target price from $25 to $22.
Furthermore, according to the comprehensive report, the opinions of $IGM Biosciences (IGMS.US)$'s main analysts recently are as follows:
IGM Biosciences has announced a shift in focus towards its I&I candidates, imvotamab and IGM-2644, while discontinuing its oncology asset aplitabart following an interim review of a study for second-line metastatic renal cell carcinoma. This strategic move comes alongside significant changes in the company's executive team concurrent with the pipeline update. The recent de-prioritization of aplitabart has led to expectations of a reduced potential for significant positive catalysts for IGM in the mid-term, resulting in an assessment that the stock currently presents an unfavorable risk/reward profile.
IGM Biosciences has announced the halting of aplitabart development, choosing to concentrate solely on autoimmune indications. This strategic shift follows the full enrollment of a randomized trial that combines aplitabart with FOLFIRI and bevacizumab in second-line metastatic colorectal cancer earlier in the year. Analysts continue to endorse the potential of bispecific T cell engagers for the treatment of B cell-driven autoimmune diseases and suggest that IgM bispecifics may offer advantages in safety and efficacy over IgG-based therapies. Consequently, aplitabart has been removed from projections, and expectations are now set for imvotamab in systemic lupus erythematosus due to the redirection of resources and focus.
Following IGM Biosciences' strategic update to concentrate solely on autoimmunity, including the ceasing of aplitabart in second-line mCRC and the prioritization of imvotamab and IGM-2644 for autoimmune indications, it's acknowledged that the discontinuation of aplitabart represents a notable challenge. However, the asset was not deemed pivotal to the core investment narrative and was recognized as a high-risk program. The pipeline update is considered an appropriate course of action and is seen as an overall positive development.
The cessation of aplitabart has been met with disappointment, yet the transition of the company into a concentrated autoimmune entity specializing in T-cell engagers is likely to clarify the investment narrative, concentrate on the most promising areas, and conserve resources. It's noted that following a decline in the stock's post-market performance, a buying opportunity is perceived in anticipation of forthcoming significant autoimmune data concerning imvotamab.
Here are the latest investment ratings and price targets for $IGM Biosciences (IGMS.US)$ from 4 analysts:
Note:
TipRanks, an independent third party, provides analysis data from financial analysts and calculates the Average Returns and Success Rates of the analysts' recommendations. The information presented is not an investment recommendation and is intended for informational purposes only.
Success rate is the number of the analyst's successful ratings, divided by his/her total number of ratings over the past year. A successful rating is one based on if TipRanks' virtual portfolio earned a positive return from the stock. Total average return is the average rate of return that the TipRanks' virtual portfolio has earned over the past year. These portfolios are established based on the analyst's preliminary rating and are adjusted according to the changes in the rating.
TipRanks provides a ranking of each analyst up to 5 stars, which is representative of all recommendations from the analyst. An analyst's past performance is evaluated on a scale of 1 to 5 stars, with more stars indicating better performance. The star level is determined by his/her total success rate and average return.
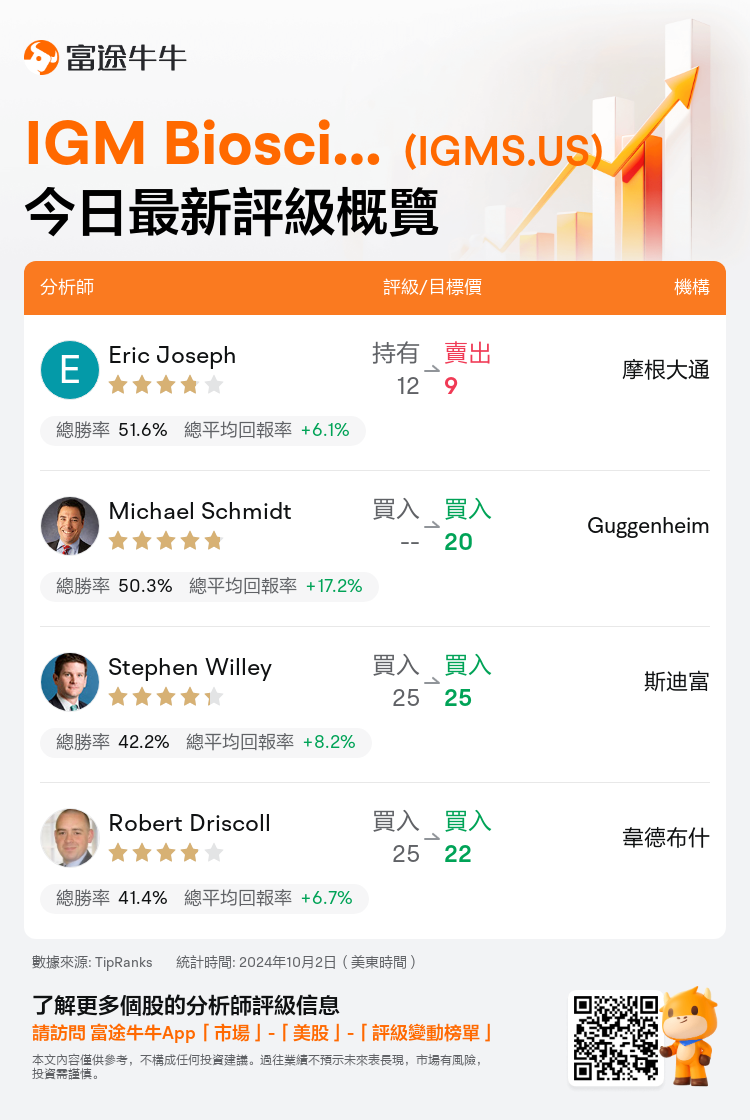
美東時間10月2日,多家華爾街大行更新了$IGM Biosciences (IGMS.US)$的評級,目標價介於9美元至25美元。
摩根大通分析師Eric Joseph下調至賣出評級,並將目標價從12美元下調至9美元。
Guggenheim分析師Michael Schmidt維持買入評級,目標價20美元。
 斯迪富分析師Stephen Willey維持買入評級,維持目標價25美元。
斯迪富分析師Stephen Willey維持買入評級,維持目標價25美元。
韋德布什分析師Robert Driscoll維持買入評級,並將目標價從25美元下調至22美元。
此外,綜合報道,$IGM Biosciences (IGMS.US)$近期主要分析師觀點如下:
IgM Biosciences宣佈將重點轉移到其I&I候選藥物imvotamab和IgM-2644上,同時在對一項二線轉移性腎細胞癌的研究進行中期審查後,停止了其腫瘤資產aplitabart。這一戰略舉措伴隨着公司高管團隊的重大變動,同時還進行了管道更新。最近取消了aplitabart的優先級,這導致人們對IgM在中期出現重大積極催化劑的可能性降低,因此評估認爲該股票目前的風險/回報狀況不佳。
IgM Biosciences宣佈停止aplitabart的開發,選擇只專注於自身免疫適應症。這一戰略轉變是在今年早些時候將阿普利塔巴特與FOLFIRI和貝伐珠單抗聯合用於二線轉移性結直腸癌的隨機試驗的全部入組之後發生的。分析人士繼續支持雙特異性T細胞參與劑治療b細胞驅動的自身免疫性疾病的潛力,並認爲與基於IgG的療法相比,IgM雙特異性可能在安全性和有效性方面具有優勢。因此,aplitabart已從預測中刪除,由於資源和重點的重定向,現已設定了對imvotamab治療系統性紅斑狼瘡的預期。
繼IgM Biosciences更新了僅專注於自身免疫的戰略更新,包括停止在二線mCRC中使用aplitabart以及優先考慮針對自身免疫適應症的imvotamab和IgM-2644之後,人們承認停止使用aplitabart是一項重大挑戰。但是,該資產不被認爲對核心投資敘事至關重要,因此被視爲高風險項目。管道更新被認爲是適當的行動方針,被視爲總體上積極的進展。
aplitabart的停止令人失望,但該公司轉變爲一家專門研究T細胞參與者的集中型自身免疫實體,很可能會澄清投資理念,專注於最有前途的領域,並節省資源。值得注意的是,在股票盤後表現下降之後,預計即將發佈有關imvotamab的重要自身免疫數據,因此看到了買入機會。
以下爲今日4位分析師對$IGM Biosciences (IGMS.US)$的最新投資評級及目標價:
提示:
TipRanks為獨立第三方,提供金融分析師的分析數據,並計算分析師推薦的平均回報率和勝率。提供的信息並非投資建議,僅供参考。本文不對評級數據和報告的完整性與準確性做出認可、聲明或保證。
TipRanks提供每位分析師的星級,分析師星級代表分析師所有推薦的過往表現,通過分析師的總勝率和平均回報率综合計算得出,星星越多,則該分析師過往表現越優異,最高爲5颗星。
分析師總勝率為近一年分析師的評級成功次數占總評級次數的比率。評级的成功與否,取決於TipRanks的虚擬投資組合是否從該股票中產生正回報。
總平均回報率為基於分析師的初始評級創建虚擬投資組合,並根據評級變化對組合進行調整,在近一年中該投資組合所獲得的回報率。
 斯迪富分析師Stephen Willey維持買入評級,維持目標價25美元。
斯迪富分析師Stephen Willey維持買入評級,維持目標價25美元。 
 Stifel analyst Stephen Willey maintains with a buy rating, and maintains the target price at $25.
Stifel analyst Stephen Willey maintains with a buy rating, and maintains the target price at $25.