Annovis Bio Announces New Data From Phase III Parkinson's Study Highlighting Improvements in Unified Parkinson's Disease Rating Scale (MDS-UPDRS) and Cognition After Treatment With Buntanetap
Annovis Bio Announces New Data From Phase III Parkinson's Study Highlighting Improvements in Unified Parkinson's Disease Rating Scale (MDS-UPDRS) and Cognition After Treatment With Buntanetap
Tue, 02 Jul 2024
2024年7月2日,星期二
Buntanetap showed dose-dependent and statistically significant improvements in cognition in the overall enrolled PD population. Parkinson's patients with substantial cognitive decline exhibited a very pronounced improvement.
Buntanetap在總體PD患者中劑量依賴地且具有統計學意義地改善了認知能力。患有明顯認知能力下降的帕金森病患者表現出了非常顯著的改善。
Buntanetap showed statistical improvement in the MDS-UPDRS Part II, Part III, Part II+III and Total scores in Parkinson's patients with a >3-year diagnosis.
Buntanetap在帕金森患者的MDS-UPDRS第II、第III、第II+III和總分數中得到了統計學改善,診斷時間超過3年。
Buntanetap showed the same statistical improvement in MDS-UPDRS Part II, Part III, Part II+III and Total scores in Parkinson's patients with Postural Instability and Gait Difficulties (PIGD).
Buntanetap在帕金森患者的姿勢不穩和步行困難(PIGD)中得到了與MDS-UPDRS第II、第III、第II+III和總分數中相同的統計改善。
Buntanetap's activity resulted in statistically significant improvements in all primary and secondary endpoints in the specified populations as well as in cognition.
Buntanetap的活性導致了所有主要和次要終點的統計學顯著改善(指定人群和認知)。Buntanetap對特定人群和認知方面的所有主要和次要終點均表現出了統計學顯著改善。
MALVERN, Pa., July 02, 2024 (GLOBE NEWSWIRE) -- via IBN -- Annovis Bio Inc. (NYSE: ANVS) ("Annovis" or the "Company"), a late-stage clinical drug platform company pioneering transformative therapies for neurodegenerative disorders such as Alzheimer's Disease (AD) and Parkinson's Disease (PD), today announced new data from its Phase III PD study demonstrating that buntanetap is safe and effective in improving motor and non-motor activities and improving cognitive functions in patients with early Parkinson's disease.
****MALVERN,2024年7月2日,環球新聞社 - 經IBN確認 -Annovis Bio公司。(NYSE:ANVS)(“Annovis”或“公司”),一家晚期臨床藥物平台公司,爲阿爾茨海默病(AD)和帕金森病(PD)等神經退行性疾病開發變革性療法,今天宣佈了其三期PD研究的新數據,證明buntanetap對早期帕金森病患者的運動和非運動活動以及認知功能具有安全且有效的改善作用。
These findings will be discussed in more detail on today's webcast at 4:30 PM ET. Register here.
這些發現將在今天下午4:30網上研討會上進行更詳細的討論。在此註冊.
"We are very pleased to see improvements in many of our patients over such a short course of treatment. These compelling data reinforce our commitment to advancing buntanetap into a longer study, which will allow us not only to verify observed symptomatic improvements but also to explore buntanetap's disease-modifying properties," said Maria Maccecchini, Ph.D., Founder, President and CEO of Annovis Bio.
“我們非常高興看到在如此短的治療過程中許多患者的改善。這些有說服力的數據加強了我們將buntanetap推至更長的研究的承諾,這將使我們不僅驗證觀察到的症狀改善,還允許我們探索buntanetap對疾病修復屬性的作用。”Annovis Bio的創始人,總裁兼首席執行官Maria Maccecchini博士說。
Key Findings from the Study:
該研究的關鍵發現結果如下:
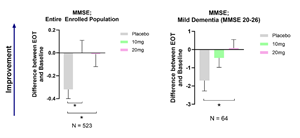
Buntanetap stops cognitive decline in all enrolled patients (MMSE 20-30) and improves cognition in patients with mild dementia (MMSE 20-26).
Buntanetap阻止了所有入組患者(MMSE 20-30)的認知能力下降,並改善了輕度癡呆患者(MMSE 20-26)的認知能力。
-
-
Findings: In the entire enrolled population, placebo group demonstrated cognition deterioration throughout the study, whereas all treatment groups (10mg and 20mg buntanetap) maintained baseline levels, indicating a statistically significant effect of the drug in stopping cognitive decline. In patients with mild dementia, as measured by MMSE 20-26, cognition deteriorated at a faster pace in the placebo group compared to those treated with 10mg buntanetap. Moreover, treatment with 20mg buntanetap showed significant improvement in cognition compared to placebo (Figure 1).
- Background: Inclusion criteria for enrollment included MMSE scores between 20-30, and MMSE scores were measured again at the end of the study as an exploratory endpoint. This allowed us to determine changes in cognition in the entire enrolled population as well as in patients with cognitive decline. Most PD patients had normal cognitive functioning, and just 12% showed cognitive decline as measured by MMSE 20-26. These patients declined by 1.5 MMSE points in the placebo group but did not decline at all and even improved when treated with buntanetap. This data aligns with our AD cognition data, where patients with MMSE>20 responded well to buntanetap, showing statistically significant cognitive improvement in early AD patients.
-
Findings: In the entire enrolled population, placebo group demonstrated cognition deterioration throughout the study, whereas all treatment groups (10mg and 20mg buntanetap) maintained baseline levels, indicating a statistically significant effect of the drug in stopping cognitive decline. In patients with mild dementia, as measured by MMSE 20-26, cognition deteriorated at a faster pace in the placebo group compared to those treated with 10mg buntanetap. Moreover, treatment with 20mg buntanetap showed significant improvement in cognition compared to placebo (Figure 1).
-
- 結果:在整個入組人群中,安慰劑組在整個研究過程中都表現出認知惡化,而所有治療組(10mg和20mg buntanetap)均保持基線水平,表明藥物在阻止認知能力下降方面具有顯著效果。對於MMSE 20-26的輕度癡呆患者,安慰劑組的認知能力下降速度比接受10mg buntanetap治療的患者快。此外,20mg buntanetap的治療與安慰劑相比在認知方面顯示出顯著改善(圖1)。
- 背景:入組標準包括MMSE評分在20-30之間,並將MMSE評分作爲勘探性終點再次測量。這使我們能夠確定整個入組人群以及認知下降患者的認知變化。大多數PD患者具有正常的認知功能,僅12%的患者表現出由MMSE20-26測量的認知功能下降。這些患者在安慰劑組中下降了1.5個MMSE點數,但在接受buntanetap治療時沒有下降,甚至有所改善。這些數據與我們的AD認知數據一致,其中MMSE> 20的患者對buntanetap作出了良好的反應,在早期AD患者中表現出了統計學上顯着的認知改善。
- 結果:在整個入組人群中,安慰劑組在整個研究過程中都表現出認知惡化,而所有治療組(10mg和20mg buntanetap)均保持基線水平,表明藥物在阻止認知能力下降方面具有顯著效果。對於MMSE 20-26的輕度癡呆患者,安慰劑組的認知能力下降速度比接受10mg buntanetap治療的患者快。此外,20mg buntanetap的治療與安慰劑相比在認知方面顯示出顯著改善(圖1)。
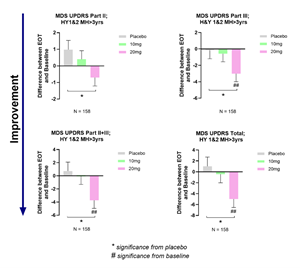
Buntanetap improves motor and non-motor PD-related functions in patients with a diagnosis of over 3 years.
Buntanetap改善了診斷時間超過3年的帕金森患者的運動和非運動PD相關功能。
-
-
Findings: Patients with a diagnosis of less than 3 years showed minimal or no deficits in MDS-UPDRS Part II, making it challenging to measure improvement and assess treatment effectiveness. However, in patients diagnosed with PD for longer than 3 years (MH>3), with measurable declines in MDS-UPDRS Part II, 20mg buntanetap significantly improved MDS-UPDRS Part II, Part III, Part II+III, and Total scores compared to placebo and baseline (Figure 2).
- Background: Longitudinal studies from Parkinson's Progressive Markers Initiative (PPMI) cohorts have shown that Parkinson patients' self-evaluation of their activities of daily living (MDS-UPDRS Part II) are relatively intact in the early disease stages. They have also shown that MDS-UPDRS Part II deteriorates at a much slower pace compared to the physician-scored motor evaluation (MDS-UPDRS Part III (Holden et al. 2017).
-
Findings: Patients with a diagnosis of less than 3 years showed minimal or no deficits in MDS-UPDRS Part II, making it challenging to measure improvement and assess treatment effectiveness. However, in patients diagnosed with PD for longer than 3 years (MH>3), with measurable declines in MDS-UPDRS Part II, 20mg buntanetap significantly improved MDS-UPDRS Part II, Part III, Part II+III, and Total scores compared to placebo and baseline (Figure 2).
-
- 結果:診斷時間少於3年的患者在MDS-UPDRS第II方面沒有或很少表現出缺陷,這使得衡量改善和評估治療效果變得具有挑戰性。然而,已經診斷爲PD超過3年(MH> 3)的患者,在MDS-UPDRS第II、第III、第II+III和總分數中,20mg buntanetap顯着改善了與安慰劑和基線(圖2)相比。
- 背景:Parkinson's Progressive Markers Initiative(PPMI)隊列的縱向研究表明,帕金森患者對其日常生活的活動的自我評估(MDS-UPDRS第II)在早期疾病階段相對完好。他們還表明,與由醫生評分的運動評估(MDS-UPDRS第III)相比,MDS-UPDRS第II以更慢的速度惡化。(Holden等人,2017年).
- 結果:診斷時間少於3年的患者在MDS-UPDRS第II方面沒有或很少表現出缺陷,這使得衡量改善和評估治療效果變得具有挑戰性。然而,已經診斷爲PD超過3年(MH> 3)的患者,在MDS-UPDRS第II、第III、第II+III和總分數中,20mg buntanetap顯着改善了與安慰劑和基線(圖2)相比。
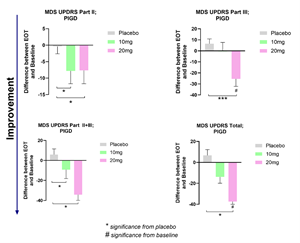
Buntanetap improves motor and non-motor PD-related functions in patients with Postural Instability and Gait Difficulty (PIGD).
Buntanetap提高了患有姿勢不穩和步行困難(PIGD)的帕金森患者的運動和非運動PD相關功能。
-
-
Findings: PIGD patients treated with buntanetap showed significant improvements in MDS-UPDRS Part II, Part III, Part II-III, and Total scores. The disease in this patient group progresses faster than in other PD patients. Our data showed that PIGD patients respond better to buntanetap and improve further than other Parkinson's patients (Figure 3).
- Background: Parkinson's is a very heterogeneous disease. Patients diagnosed with PIGD are considered to have faster disease progression (Jankovic et al. 1990 & Stebbins et al. 2013) than patients with no postural and gait issues. This observation provided us with the rationale to evaluate whether PIGD patients, who are at risk of faster decline, would benefit from buntanetap.
-
Findings: PIGD patients treated with buntanetap showed significant improvements in MDS-UPDRS Part II, Part III, Part II-III, and Total scores. The disease in this patient group progresses faster than in other PD patients. Our data showed that PIGD patients respond better to buntanetap and improve further than other Parkinson's patients (Figure 3).
-
- 結果:接受buntanetap治療的PIGD患者在MDS-UPDRS第II、第III、第II-III和總分數方面表現出顯着改善。這種患者組中的疾病進展速度比其他PD患者快。我們的數據顯示,PIGD患者對buntanetap的反應更好,並且比其他帕金森患者有進一步的改善(圖3)。
- 背景:帕金森病是一種非常多樣化的疾病。被診斷爲PIGD的患者被認爲具有更快的疾病進展(19)比沒有姿勢和步行問題的患者更高。這種觀察使我們有理由評估是否PIGD患者(有較快的衰退風險)將從buntanetap中受益。(Jankovic等人,1990& Stebbins等人,2013)
- 結果:接受buntanetap治療的PIGD患者在MDS-UPDRS第II、第III、第II-III和總分數方面表現出顯着改善。這種患者組中的疾病進展速度比其他PD患者快。我們的數據顯示,PIGD患者對buntanetap的反應更好,並且比其他帕金森患者有進一步的改善(圖3)。
Safety Profile: Buntanetap maintained a consistent safety profile across all participants, with no significant differences between early and advanced PD patients confirming our previous AD data.
安保立單點注射在所有病人中,包括PD早期和晚期患者,保持了一致的安全檔案,確認了我們之前AD數據的無顯著差異。
FDA and Endpoint Clarification: Recent questions regarding the primary and secondary endpoints in MDS-UPDRS scores warrants additional explanation. Initially, Annovis chose MDS-UPDRS Part II+III as the primary endpoint. However, based on FDA feedback, MDS-UPDRS Part II alone was deemed more appropriate for reflecting clinically relevant changes (Goetz et al., 2023). Consequently, we adjusted our primary endpoint to MDS-UPDRS Part II, with MDS-UPDRS Part III as a key secondary endpoint. Our results met both primary and secondary endpoints in the specified subgroups.
FDA和終點清晰化:最近有關MDS-UPDRS積分的主要和次要終點的問題需要更多解釋。最初,安保立選擇MDS-UPDRS第II+III部分爲主要終點。然而,根據FDA的反饋,僅使用MDS-UPDRS第II部分更適合反映臨床相關變化(Goetz等人,2023年)。因此,我們將主要終點調整爲MDS-UPDRS第II部分,MDS-UPDRS第III部分成爲重要的次要終點。我們的結果在指定的亞組中達到了主要和次要終點。
Upcoming Webcast
Annovis Bio will host an investor webcast today at 4:30 PM ET to discuss these findings in detail. Investors and interested parties are encouraged to register for the webcast in advance.
To register, please visit Webcast Registration and complete the registration form.
即將來臨的網絡研討會
Annovis Bio將於今天下午4:30舉行投資者網絡研討會,詳細討論這些調查結果。鼓勵投資者和有興趣方在研討會之前提前註冊。
要註冊,請訪問網絡研討會註冊關於Buntanetap: Buntanetap(以前稱爲Posiphen或ANVS401)通過抑制多種神經毒蛋白的形成,包括澱粉樣蛋白β、tau、α-突觸核蛋白和TDP43,以改善突觸傳遞、軸索轉運和減少神經炎症,旨在逆轉AD、PD和其他神經退行性疾病的神經退變現象。
About Buntanetap
Buntanetap (formerly known as Posiphen or ANVS401) targets neurodegeneration by inhibiting the formation of multiple neurotoxic proteins, including amyloid beta, tau, alpha-synuclein, and TDP43. By improving synaptic transmission, axonal transport, and reducing neuroinflammation, Buntanetap aims to reverse neurodegeneration in AD, PD, and other neurodegenerative diseases, thereby aiming to restore brain function and improve the quality of life for patients.
關於安保立
安保立(曾用名Posiphen或ANVS401)通過抑制多種神經毒性蛋白,包括澱粉樣β、tau、α-突觸核蛋白和TDP43的形成,以針對神經退行性疾病。通過改善突觸傳輸、軸突輸運和減少神經炎症,安保立旨在逆轉AD、PD和其他神經退行性疾病的神經退行,從而恢復大腦功能,並提高患者的生活質量。
References
參考
- Holden, SK, Finseth, T, Sillau, SH and Berman, BD. Progression of MDS-UPDRS Scores Over Five Years in De Novo Parkinson Disease from the Parkinson's Progression Markers Initiative Cohort. Mov Disord Clin Pract 2018, 5: 47-53. [PubMed]
- Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson's disease: a base‐line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 1990; 40:1529–1534. [PubMed] [Google Scholar]
- Stebbins GT, Goetz CG, Burn DJ, et al. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson's disease rating scale: comparison with the unified Parkinson's disease rating scale. Mov Disord 2013; 28:668–670. [PubMed] [Google Scholar]
- Goetz, CG, Choi, D., Guo, Y., Stebbins, GT, Mestre, TA., & Luo, S. It Is as It Was: MDSUPDRS Part III Scores Cannot Be Combined with Other Parts to Give a Valid Sum. Movement Disorders 2023, 38(2), 342-347. [PubMed]
- Holden,SK、Finseth,T、Sillau,SH和Berman,BD。在來自Parkinson's Progression Markers Initiative Cohort的De Novo Parkinson Disease患者中,五年內MDS-UPDRS積分的進展。Mov Disord Clin Pract 2018,5:47-53。[PubMed]
- Jankovic J, McDermott M, Carter J, et al. Parkinson's disease: a baseline analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 1990;40:1529–1534。[‐PubMed Google學者斯特賓斯GT、高澄、Burn DJ等。如何使用運動障礙學會統一的帕金森病評分表識別震顫優勢和姿勢不穩/步態困難組:與統一的帕金森病評分表相比較。運動障礙1993年;28:668-670。[] [PubMed]
- 谷大偉,高珊軍,Steppins GT,Mestre TA以及羅蘇。它與過去一樣:MDSUPDRS第III部分的得分不能與其他部分相結合來給出有效的總和。運動障礙2023,38(2),342-347。[PubMed] [Google學者]
- 變速器其他]
About Annovis Bio, Inc.
Headquartered in Malvern, Pennsylvania, Annovis Bio Inc. is dedicated to addressing neurodegeneration in diseases such as AD and PD. The company's innovative approach targets multiple neurotoxic proteins, aiming to restore brain function and improve the quality of life for patients. For more information, visit www.annovisbio.com and follow us on LinkedIn, YouTube, and X.
Annovis Bio,Inc.
總部位於賓夕法尼亞州馬爾文的Annovis Bio Inc.致力於解決AD和PD等疾病中的神經退行問題。該公司的創新方法針對多種神經毒素蛋白,旨在恢復大腦功能並提高患者的生活質量。www.annovisbio.com和我們一起LinkedIn, YouTube和X.
Interested investors and shareholders are encouraged to sign up for press releases and industry updates by registering for Email Alerts at https://www.Annovisbio.com/email-alerts
鼓勵感興趣的投資者和股東通過註冊電子郵件提醒來註冊以獲取新聞發佈和行業更新。https://www.Annovisbio.com/email-alerts
Forward-Looking Statements
This press release contains "forward-looking" statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements include, but are not limited to, the Company's plans related to clinical trials. Forward-looking statements are based on current expectations and assumptions and are subject to risks and uncertainties that could cause actual results to differ materially from those projected. Such risks and uncertainties include, but are not limited to, those related to patient enrollment, the effectiveness of buntanetap, and the timing, effectiveness, and anticipated results of the Company's clinical trials evaluating the efficacy, safety, and tolerability of buntanetap. Additional risk factors are detailed in the Company's periodic filings with the SEC, including those listed in the "Risk Factors" section of the Company's Annual Report on Form 10-K and Quarterly Reports on Form 10-Q. All forward-looking statements in this press release are based on information available to the Company as of the date of this release. The Company expressly disclaims any obligation to update or revise its forward-looking statements, whether as a result of new information, future events, or otherwise, except as required by law.
前瞻性聲明
本新聞稿包含根據美國證券法第27A條修正案和證券交易法第21E條修正案進行的"前瞻性"聲明。 這些聲明包括但不限於公司與臨床試驗相關的計劃。前瞻性聲明基於當前的期望和假設,並且可能受到風險和不確定性的影響,這些風險和不確定性可能會導致實際結果與預期結果不同。 此類風險和不確定性包括但不限於患者入組情況,buntanetap 的有效性以及公司評估 buntanetap 療效,安全性和耐受性的臨床試驗的時間,有效性和預期結果。 其他風險因素請參見公司向證券交易委員會提交的定期報告,包括在公司年度報告的“風險因素”部分和每季度報告的表格10-Q中列出的風險因素。 本新聞稿中的所有前瞻性聲明都基於公司在發佈日期可獲得的信息。 除非法律規定,否則本公司明確不承擔更新或修訂其前瞻性聲明的義務。
Contacts
Annovis Bio, Inc.
101 Lindenwood Drive
Suite 225
Malvern, PA 19355
www.annovisbio.com
聯繫方式
Annovis Bio, Inc.
101 Lindenwood Drive
225套房
馬爾文,PA 19355
www.annovisbio.com
Investor Contact
Scott McGowan
InvestorBrandNetwork (IBN)
Phone: 310.299.1717
IR@annovisbio.com
Investor Website
投資者聯繫方式
Scott McGowan
InvestorBrandNetwork(IBN)
電話:310.299.1717
IR@annovisbio.com
投資者網站
Attachments
附件
- Changes in MMSE after treatment with 10mg or 20mg buntanetap compared to baseline and placebo
- Changes in MDS-UPDRS after treatment with 10mg or 20mg buntanetap in patients with a PD diagnosis over 3 years compared to baseline and placebo
- Changes in MDS-UPDRS after treatment with 10mg or 20mg buntanetap in patients with PIGD
Changes in MMSE after treatment with 10mg or 20mg buntanetap compared to baseline and placebo
治療期間使用10mg或20mg的布坦尼泰普與基線和安慰劑相比,MMSE發生變化
Figure 1.
圖1。
Changes in MDS-UPDRS after treatment with 10mg or 20mg buntanetap in patients with a PD diagnosis over 3 years compared to baseline and placebo
在3年以上被診斷爲PD的患者中,使用10mg或20mg布坦尼泰普治療後,MDS-UPDRS與基線和安慰劑相比發生變化
Figure 2.
圖2。
Changes in MDS-UPDRS after treatment with 10mg or 20mg buntanetap in patients with PIGD
在PIGD患者中,使用10mg或20mg布坦尼泰普治療後,MDS-UPDRS發生變化
Figure 3.
圖3。
Source: Annovis Bio, Inc.
消息來源:annovis bio,inc.
譯文內容由第三人軟體翻譯。