XBiotech Results From Randomized Double-Blinded Phase 1/2 Study Suggest Potential Breakthrough Treatment for Advanced Pancreatic Cancer
XBiotech Results From Randomized Double-Blinded Phase 1/2 Study Suggest Potential Breakthrough Treatment for Advanced Pancreatic Cancer
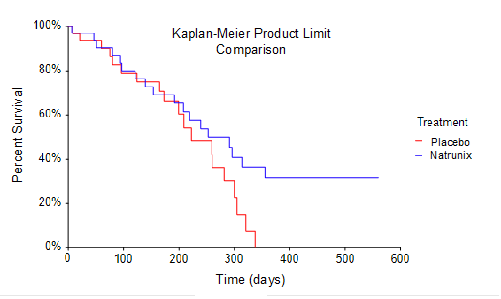
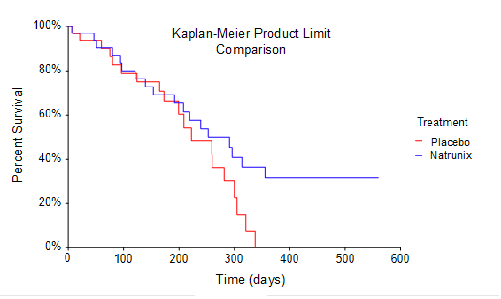
Overall Survival (OS) defined as from time of randomization to death. Natrunix + ON+5FU+LV (n=3) vs Placebo + ON+5FU+LV arms (n=32) were analyzed in a Kaplan-Meier Survival plot using a product limit comparison method. Borderline statistically significance of p = 0.096, given the small sample size for survival analysis, suggests prolonged survival for subjects receiving Natrunix.
總體存活率(OS)定義爲從隨機分組到死亡。使用產品極限比較方法在卡普蘭-邁爾生存圖中分析了Natrunix + ON+5FU+LV(n=3)與安慰劑 + ON+5FU+LV 雙臂(n=32)的對比。鑑於存活分析的樣本量很小,p = 0.096的臨界統計學意義表明接受Natrunix治療的受試者的存活時間延長。
Findings Show Trends for Reduced Toxicities and Better Outcomes for Subjects Receiving ONIVYDE/5-FU Combination and Targeted anti-IL-1alpha Therapy
研究結果顯示,接受ONIVYDE/5-FU組合和靶向抗IL-1α療法的受試者有降低毒性和改善療效的趨勢
AUSTIN, Texas, June 18, 2024 (GLOBE NEWSWIRE) -- XBiotech (NASDAQ: XBIT) announced today data from its Phase 1/Phase 2 randomized, double-blind, placebo-controlled multi-center study for advanced pancreatic cancer. Known as 1-BETTER, the study examined Natrunix (anti-interleukin-1alpha) antibody in combination with an established chemotherapy regimen (ONIVYDE (ON) + 5-Fluorouracil (5FU) + Leucovorin (LV), a regimen that is already widely used for treating pancreatic cancer but is associated with difficult toxicities and less then ideal survival outcomes. Natrunix was being evaluated as an anti-cancer agent for use in cytotoxic chemotherapy combinations where the Company believes it might potentially also improve tolerability of the chemotherapy.
得克薩斯州奧斯汀,2024年6月18日(GLOBE NEWSWIRE)——xBIOTECH(納斯達克股票代碼:XBIT)今天公佈了其針對晚期胰腺癌的1/2期隨機、雙盲、安慰劑對照的多中心研究的數據。這項名爲1-BETTER的研究考察了Natrunix(抗白介素-1α)抗體與既定的化療方案(ONIVYDE(ON)+ 5-氟尿嘧啶(5FU)+ Leucovorin(LV)的聯合療法,該方案已被廣泛用於治療胰腺癌,但毒性很大,存活結果不太理想。Natrunix被評估爲一種用於細胞毒性化療組合的抗癌藥物,該公司認爲它還可能提高化療的耐受性。
The Phase 1 portion was a dose escalation study in metastatic pancreatic cancer patients to determine if dose limiting toxicities (DLTs) occurred in combination with the ON+5FU+LV regimen in second- or third-line setting. DLTs were not expected with Natrunix and none were seen. The Natrunix dose in the Phase 2 portion was thus the highest dose used in the Phase 1 portion.
第一階段是針對轉移性胰腺癌患者的劑量遞增研究,旨在確定在二線或三線環境中與ON+5FU+LV方案聯合使用時是否出現劑量限制毒性(DLT)。預計 Natrunix 不會有 DLT,也沒有看到任何東西。因此,第二階段部分的Natrunix劑量是第一階段使用的最高劑量。
Sixty-five subjects were randomized into the Phase 2 study on a 1:1 basis to receive either Natrunix+ ON+5FU+LV (Arm1) or Placebo +ON+5FU+LV (Arm2). There were 33 subjects enrolled into Arm1 and 32 into Arm2. The Phase 2 treatment period was 24-weeks with subjects receiving therapy once every other week for a total of 12 cycles.
65名受試者以 1:1 的比例隨機進入第二階段研究,接受Natrunix+ ON+5FU+LV(Arm1)或安慰劑+ON+5FU+LV(Arm2)治療。有33名受試者加入了Arm1,32名受試者加入了Arm2。第二階段治療期爲24周,受試者每隔一週接受一次治療,共12個週期。
Subjects included in the study had confirmed metastatic, unresectable, or recurrent pancreatic adenocarcinoma of exocrine pancreas and were required to have had disease progression after one prior gemcitabine-based therapy or one FOLFIRINOX and gemcitabine containing therapy. All patients were required to have at least one measurable lesion according to Response Evaluation Criteria in Solid Tumor (RECIST v1.1).
該研究中包括的受試者已證實胰腺外分泌轉移性、不可切除的或復發的胰腺腺癌,並且必須在先前接受過一次基於吉西他濱的治療或一次含有FOLFIRINOX和吉西他濱的治療後出現疾病進展。根據實體瘤反應評估標準(RECIST v1.1),所有患者都必須有至少一個可測量的病變。
The primary endpoint for the Phase 2 study was to assess the safety and tolerability of Natrunix when used with the ON+5FU+LV combination. Overall, there were fewer adverse events (AEs) of any kind during the 24-week treatment period for the Natrunix arm compared to placebo (297 vs 336), with markedly fewer events in specific categories of adverse events during that time. There was a 28% reduction in the number of subjects experiencing significant adverse events (SAEs) in the Natrunix arm (9 out of 33) versus placebo (12 out of 32) that occurred during the 24-week treatment period. Subjects receiving the Natrunix ON+5FU+LV regimen also had about a 33% reduction in hospitalization (80 days versus 120 days) during the 24-week treatment period compared to subjects receiving placebo + ON+5FU+LV combination.
2期研究的主要終點是評估Natrunix與ON+5FU+LV組合使用時的安全性和耐受性。總體而言,與安慰劑相比,在24周的治療期內,Natrunix組的任何種類的不良事件(AE)都較少(297對比336),在此期間,特定類別的不良事件明顯減少。與在24周治療期內發生的安慰劑(32人中有12人)相比,Natrunix組出現重大不良事件(SAE)的受試者(33人中有9人)減少了28%。與接受安慰劑+ ON+5FU+LV聯合治療的受試者相比,在24周的治療期內,接受Natrunix ON+5FU+LV方案的受試者的住院時間也減少了約33%(80天對比120天)。
Subjects receiving the Natrunix combination also reported a 22% reduction in fatigue (28 vs 36), 32% improved appetite (19 vs 28) and 41% reduction in pain (17 vs 29) as of the last day of the 24-week treatment period compared to subjects receiving the placebo ON+5FU+LV combination.
接受Natrunix組合的受試者還報告說,與接受安慰劑ON+5FU+LV組合的受試者相比,截至24周治療期的最後一天,疲勞減輕了22%(28對36),食慾改善了32%(19對28),疼痛減輕了41%(17對29)。
Severe diarrhea that can be life -threatening is a significant complication for the ON+5FU+LV regimen. There was a two-fold reduction (9% versus 19%) in the incidence of severe diarrhea during the 24-week treatment regimen for patients receiving the Natrunix + ON+5FU+LV combination compared to placebo + ON+5FU+LV.
可能危及生命的嚴重腹瀉是ON+5FU+LV方案的重大併發症。與安慰劑+ ON+5FU+LV相比,在爲期24周的治療方案中,接受Natrunix + ON+5FU+LV組合的患者的嚴重腹瀉發病率降低了兩倍(9%對19%)。
Overall Survival (OS), one of the secondary endpoints for the Phase 2 study, was conventionally defined in as time from randomization to death. The sample size for the study included intent-to-treat analysis of 33 subjects randomized into the Natrunix + ON+5FU+LV arm versus 32 subjects in Placebo + ON+5FU+LV arm. A Kaplan-Meier Survival Curve using a product limit comparison method was performed. This data highlights the observation that no subjects in the placebo ON+5FU+LV group (n=32) survived for longer than 330 days, whereas 8 subjects in the Natrunix ON+5FU+LV arm (n=33) were still alive as of day 330. Considering the small sample size, the borderline statistically significant p-value of p = 0.096 suggests prolonged survival for subjects receiving the Natrunix regimen.
總體存活率(OS)是2期研究的次要終點之一,傳統上定義爲從隨機分組到死亡的時間。該研究的樣本量包括對33名隨機進入Natrunix + ON+5FU+LV組的受試者的治療意向分析,而安慰劑+ ON+5FU+LV組的32名受試者的治療意向分析。使用產品極限比較方法繪製了卡普蘭-邁爾生存曲線。這些數據突顯了這樣的觀察,即安慰劑ON+5FU+LV組(n=32)中沒有受試者的存活時間超過330天,而截至第330天,Natrunix ON+5FU+LV組(n=33)中有8名受試者還活着。考慮到樣本量小,具有統計學意義的臨界值 p = 0.096 表明接受 Natrunix 方案的受試者的存活時間延長。
The lead investigator for the study, David J. Park, MD Medical Oncologist, Medical Director for the providence St. Jude Crosson Institute, Fullerton, CA stated "Treatment of advanced pancreatic cancer in the second and third line settings presents significant challenges in terms of toxicity as well as efficacy. To observe these trends for reduced toxicity and potential survival benefit is remarkable, particularly given the limited sample size. The potential interaction between reduced toxicity, more time on treatment and improvement in survival makes intuitive sense for clinicians who treat these patients. These findings are extremely important."
該研究的首席研究員、加利福尼亞州富樂頓普羅維登斯聖裘德·克羅森研究所醫學主任腫瘤內科醫生大衛·帕克表示:“在二線和三線環境中治療晚期胰腺癌在毒性和療效方面都存在重大挑戰。觀察這些毒性降低和潛在生存益處的趨勢是顯著的,尤其是在樣本量有限的情況下。對於治療這些患者的臨床醫生來說,減少毒性、延長治療時間和提高存活率之間的潛在相互作用在直觀上是合理的。這些發現非常重要。”
While there was a relatively small number of pancreatic cancer patients enrolled in the Phase 2 portion of the study, in the Company's opinion, the findings show better outcomes for the Natrunix + ON+5FU+LV group as compared to the control arm. The Company believes that the reduced number of serious and adverse events, the significant reduction in hospitalization, and improved OS during the respective time periods described above for each of these metrics suggest that Natrunix could represent a breakthrough advance for the treatment of pancreatic cancer.
該公司認爲,儘管參與該研究第二階段的胰腺癌患者人數相對較少,但研究結果表明,與對照組相比,Natrunix + ON+5FU+LV組的預後更好。該公司認爲,在上述每項指標的相應時間段內,嚴重和不良事件數量的減少、住院人數的顯著減少以及操作系統的改善,表明Natrunix可能代表胰腺癌治療的突破性進展。
About XBiotech
XBiotech is pioneering the discovery and development of targeted antibodies based on its True Human technology. The company's mission is to rethink the way antibody medicines are discovered and commercialized by advancing its robust pipeline of truly natural human antibodies for treating serious diseases such as inflammatory conditions like rheumatology, infectious disease, cardiovascular disease and cancer. XBiotech has several candidate products including Natrunix. Cloned from individual donors who possess natural immunity against certain targeted diseases, XBiotech's pipeline of True Human antibodies are intended to deliver unmatched safety and efficacy. Located just minutes from downtown Austin, the XBiotech campus headquarters includes GMP manufacturing facilities, research and testing laboratories, infectious disease research facilities, and quality control and clinical operations. For more information, visit www.xbiotech.com.
關於 xBiotech
xBiotech在基於其真人技術的基礎上率先發現和開發靶向抗體。該公司的使命是通過推進其強大的真正天然的人體抗體產品線,重新思考抗體藥物的發現和商業化的方式,這些抗體藥物用於治療風溼病、傳染病、心血管疾病和癌症等炎症性疾病。xBiotech有幾種候選產品,包括Natrunix。xBiotech的True Human抗體產品線從對某些靶向疾病具有自然免疫力的個人捐贈者中克隆而來,旨在提供無與倫比的安全性和有效性。xBiotech園區總部距奧斯汀市中心僅數分鐘路程,包括GMP製造設施、研究和測試實驗室、傳染病研究設施以及質量控制和臨床運營。欲了解更多信息,請訪問 www.xbiotech.com.
Cautionary Note on Forward-Looking Statements and Study Results
This press release contains forward-looking statements, including declarations regarding management's beliefs and expectations that involve substantial risks and uncertainties. Forward-looking statements are subject to inherent risks and uncertainties in predicting future results and conditions that could cause the actual results to differ materially from those projected in these forward-looking statements. These risks and uncertainties are subject to the disclosures set forth in the "Risk Factors" section of certain of our SEC filings. Any forward-looking statements that we make in this press release speak only as of the date of this press release. We assume no obligation to update our forward-looking statements whether as a result of new information, future events or otherwise, after the date of this press release. The Company makes no representations regarding OS or any other metric beyond the time periods specifically discussed herein. There can be no assurance that any study results discussed in this press release will be replicated in future studies or that Natrunix will be approved by the Food and Drug Administration or any other regulator.
關於前瞻性陳述和研究結果的警示說明
本新聞稿包含前瞻性陳述,包括有關管理層信念和期望的聲明,這些聲明涉及重大風險和不確定性。前瞻性陳述在預測未來業績和條件時存在固有的風險和不確定性,這些風險和不確定性可能導致實際結果與這些前瞻性陳述中的預測存在重大差異。這些風險和不確定性受我們在美國證券交易委員會某些文件的 “風險因素” 部分中披露的約束。我們在本新聞稿中所作的任何前瞻性陳述僅代表截至本新聞稿發佈之日。在本新聞稿發佈之日之後,無論是由於新信息、未來事件還是其他原因,我們都沒有義務更新我們的前瞻性陳述。除了本文特別討論的時間段外,公司對操作系統或任何其他指標不作任何陳述。無法保證本新聞稿中討論的任何研究結果將在未來的研究中複製,也無法保證Natrunix將獲得美國食品藥品監督管理局或任何其他監管機構的批准。
Contact
聯繫我們
Wenyi Wei
wwei@xbiotech.com
Tel. 737-207-4600
魏文義
wwei@xbiotech.com
電話:737-207-4600
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/7a17b3d3-b304-47ae-b1e7-0950f0301f12
譯文內容由第三人軟體翻譯。

 Sixty-five subjects were randomized into the Phase 2 study on a 1:1 basis to receive either Natrunix+ ON+5FU+LV (Arm1) or Placebo +ON+5FU+LV (Arm2). There were 33 subjects enrolled into Arm1 and 32 into Arm2. The Phase 2 treatment period was 24-weeks with subjects receiving therapy once every other week for a total of 12 cycles.
Sixty-five subjects were randomized into the Phase 2 study on a 1:1 basis to receive either Natrunix+ ON+5FU+LV (Arm1) or Placebo +ON+5FU+LV (Arm2). There were 33 subjects enrolled into Arm1 and 32 into Arm2. The Phase 2 treatment period was 24-weeks with subjects receiving therapy once every other week for a total of 12 cycles.