Genprex Collaborators Publish Positive Preclinical Data With NPRL2 Gene Therapy Utilizing Oncoprex Delivery System
Genprex Collaborators Publish Positive Preclinical Data With NPRL2 Gene Therapy Utilizing Oncoprex Delivery System
NPRL2 Gene Therapy Induces Anti-Tumor Activity in Anti-PD1 Resistant KRAS/STK11 Mutant Non-Small Cell Lung Cancer in a Humanized Mouse Model
NPRL2 基因療法在人源化小鼠模型中誘導抗抗 PD1 的 KRAS/STK11 突變體非小細胞肺癌的抗腫瘤活性
Provides Additional Preclinical Validation of Oncoprex Delivery System With Another Tumor Suppressor Gene
使用另一種腫瘤抑制基因對 Oncoprex 遞送系統提供額外的臨床前驗證
AUSTIN, Texas — (April 2, 2024) — Genprex, Inc. ("Genprex" or the "Company") (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced that its research collaborators have published positive preclinical data for the NPRL2 tumor suppressor gene, utilizing the Company's non-viral Oncoprex Delivery System, in KRAS/STK11 mutant anti-PD1 resistant non-small cell lung cancer (NSCLC) in a humanized mouse model.
德克薩斯州奧斯汀——(2024年4月2日)— Genprex, Inc. (“Genprex” 或 “公司”)(納斯達克: GNPX)是一家專注於爲癌症和糖尿病患者開發改變生活的療法的臨床階段基因療法的公司,今天宣佈,其研究合作者已經利用公司的非病毒Oncoprex遞送系統,在人化小鼠模型中公佈了KRAS/STK11突變體抗PD1耐藥非小細胞肺癌(NSCLC)中 NPRL2 腫瘤抑制基因的積極臨床前數據。
NPRL2 is a tumor suppressor gene whose expression is reduced in many cancers including lung, renal, colorectal, glioma, gastric, and hepatocellular carcinoma, and it has been closely correlated with poor clinical outcomes.
NPRL2 是一種腫瘤抑制基因,其在許多癌症中的表達會降低,包括肺癌、腎癌、結直腸癌、神經膠質瘤、胃癌和肝細胞癌,它與不良的臨床預後密切相關。
Genprex's Oncoprex Delivery System is a novel non-viral approach that utilizes lipid-based nanoparticles in a lipoplex form to deliver tumor suppressor genes deleted during the course of cancer development. The platform allows for the intravenous delivery of various tumor suppressor genes, and potentially other genes, to achieve a therapeutic affect without the risk of toxicity often associated with viral delivery systems. Genprex believes this system allows for delivery of a number of cancer-fighting genes, alone or in combination with other cancer therapies, to combat multiple types of cancer.
Genprex 的 Oncoprex 輸送系統是一種新型的非病毒方法,它利用脂質體形式的脂質納米顆粒來輸送癌症發育過程中被刪除的腫瘤抑制基因。該平台允許靜脈注射各種腫瘤抑制基因,可能還有其他基因,以達到治療效果,而不會出現通常與病毒遞送系統相關的毒性風險。Genprex認爲,該系統允許單獨或與其他癌症療法聯合傳遞多種抗癌基因,以對抗多種類型的癌症。
The manuscript, titled, "NPRL2 gene therapy induces effective anti-tumor immunity in KRAS/STK11 mutant anti-PD1 resistant metastatic non-small cell lung cancer (NSCLC) in a humanized mouse model," was published on the bioRxiv biology preprint server.
這份手稿的標題是”NPRL2 基因療法可誘導人源化小鼠模型中 KRAS/STK11 突變體抗 PD1 耐藥轉移性非小細胞肺癌 (NSCLC) 的有效抗腫瘤免疫,” 發表在bioRxiv生物學預印本服務器上。
"These positive preclinical data are very encouraging and support NPRL2 gene therapy as a potential treatment for a sub-group of NSCLC in which patients traditionally are resistant to existing therapies," said Rodney Varner, President, Chairman and Chief Executive Officer at Genprex. "We believe this data could support the potential for a new drug candidate in our pipeline, and it also provides further evidence that the Oncoprex Delivery System has the ability to be successful using genes other than the TUSC2 gene we are already using in clinical trials with Reqorsa."
Genprex總裁、董事長兼首席執行官羅德尼·瓦納表示:“這些積極的臨床前數據非常令人鼓舞,支持將NPRL2 基因療法作爲傳統患者對現有療法具有耐藥性的非小細胞肺癌亞組的潛在治療方法。”“我們認爲,這些數據可以支持我們研發的新候選藥物的潛力,還提供了進一步的證據,表明Oncoprex遞送系統能夠成功使用我們在Reqorsa臨床試驗中已經使用的 TUSC2 基因以外的基因。”
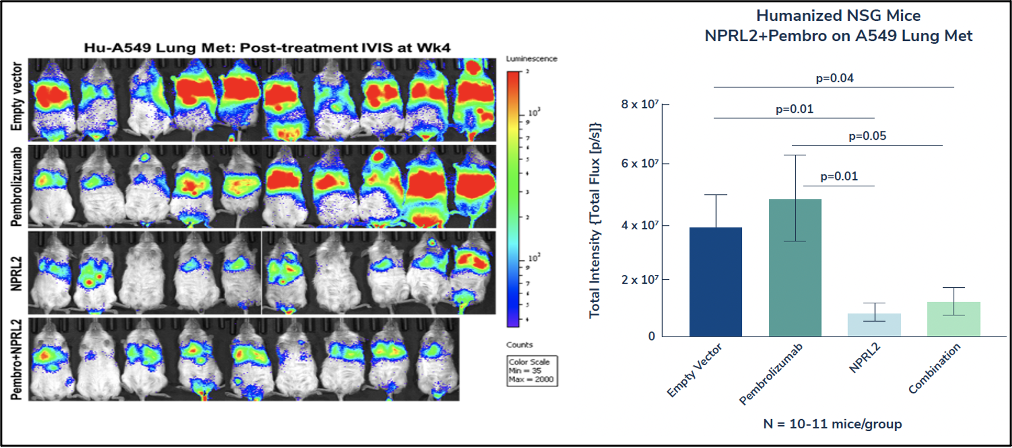
The studies evaluated the intravenous injection of NPRL2 gene-loaded cationic lipoplexes (DOTAP-NPRL2) with or without anti-PD1 drugs (pembrolizumab). The studies used a KRAS/STK11 mutant anti-PD1 insensitive cell line, as well as syngeneic mouse LLC2 tumors, which are also anti-PD1 resistant. In both of these mouse models, NPRL2 showed a significantly strong anti-tumor effect whereas anti-PD1 (pembrolizumab) was not effective. The anti-tumor effect was greater in humanized mice than non-humanized mice, suggesting that an immune response contributed to anti-tumor activity.
這些研究評估了靜脈注射含有或不含抗 PD1 藥物(pembrolizumab)的 NPRL2 基因陽離子脂肪酸(DOTAP-NPRL2)。這些研究使用了KRAS/STK11突變體抗PD1不敏感細胞系,以及同樣具有抗PD1耐藥性的同源小鼠LLC2腫瘤。在這兩個小鼠模型中,NPRL2 顯示出明顯的強大抗腫瘤作用,而抗PD1(pembrolizumab)無效。人源化小鼠的抗腫瘤作用比非人源化小鼠更大,這表明免疫反應有助於抗腫瘤活性。
Additionally, a dramatic anti-tumor effect was mediated by NPRL2 treatment with or without a pembrolizumab combination. Bioluminescence imaging on mice showed that 7 out of 10 mice contained an extremely low amount of tumor burden in the NPRL2 treatment group, which was significantly different than in the control or pembrolizumab group.
此外,無論是否使用pembrolizumab聯合用藥,NPRL2 治療都介導了顯著的抗腫瘤作用。對小鼠的生物發光成像顯示,在 NPRL2 治療組中,十分之七的小鼠的腫瘤負擔極低,這與對照組或pembrolizumab組顯著不同。
Unlike previous experiments with Reqorsa Immunogene Therapy (quartusugene ozeplasmid), the Company's lead drug candidate using the TUSC2 tumor suppressor gene, the anti-tumor efficacy of DOTAP-NPRL2 did not involve Natural Killer (NK) cells. The studies also found that tumors with stable NPRL2 expression exhibited significantly slower growth compared to controls. In conclusion, researchers reported that NPRL2 gene therapy induces anti-tumor activity through dendritic cell-mediated antigen presentation and cytotoxic immune cell activation.
與之前使用該公司使用 TUSC2 腫瘤抑制基因的主要候選藥物Reqorsa免疫基因療法(quartusugene ozeplasmid)的實驗不同,DOTAP-NPRL2 的抗腫瘤功效不涉及自然殺傷(NK)細胞。研究還發現,與對照組相比,NPRL2 表達穩定的腫瘤的生長明顯放緩。總之,研究人員報告說,NPRL2 基因療法通過樹突狀細胞介導的抗原呈現和細胞毒性免疫細胞激活來誘導抗腫瘤活性。
Genprex, Inc. is a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes. Genprex's technologies are designed to administer disease-fighting genes to provide new therapies for large patient populations with cancer and diabetes who currently have limited treatment options. Genprex works with world-class institutions and collaborators to develop drug candidates to further its pipeline of gene therapies in order to provide novel treatment approaches. Genprex's oncology program utilizes its systemic, non-viral Oncoprex Delivery System which encapsulates the gene-expressing plasmids using lipid-based nanoparticles in a lipoplex form. The resultant product is administered intravenously, where it is taken up by tumor cells that then express tumor suppressor proteins that were deficient in the tumor. The Company's lead product candidate, Reqorsa Immunogene Therapy (quaratusugene ozeplasmid), is being evaluated in three clinical trials as a treatment for NSCLC and SCLC. Each of Genprex's three lung cancer clinical programs has received a Fast Track Designation from the FDA for the treatment of that patient population, and Genprex's SCLC program has received an FDA Orphan Drug Designation. Genprex's diabetes gene therapy approach is comprised of a novel infusion process that uses an AAV vector to deliver Pdx1 and MafA genes directly to the pancreas. In models of Type 1 diabetes, GPX-002 transforms alpha cells in the pancreas into functional beta-like cells, which can produce insulin but may be distinct enough from beta cells to evade the body's immune system. In a similar approach, GPX-002 for Type 2 diabetes, where autoimmunity is not at play, is believed to rejuvenate and replenish exhausted beta cells.
Genprex, Inc. 是一家臨床階段的基因療法公司,專注於爲癌症和糖尿病患者開發改變生活的療法。Genprex的技術旨在管理抗病基因,爲目前治療選擇有限的大量癌症和糖尿病患者群體提供新療法。Genprex與世界一流的機構和合作者合作,開發候選藥物,以進一步發展其基因療法產品線,從而提供新的治療方法。Genprex的腫瘤學項目利用其全身性非病毒Oncoprex輸送系統,該系統使用脂質基納米顆粒以脂質形式封裝表達基因的質粒。所得產物通過靜脈注射,由腫瘤細胞吸收,然後腫瘤細胞表達腫瘤中缺乏的腫瘤抑制蛋白。該公司的主要候選產品Reqorsa免疫基因療法(quaratusugene ozeplasmid)正在三項臨床試驗中作爲非小細胞肺癌和小細胞肺癌的治療方法接受評估。Genprex的三個肺癌臨床項目均已獲得美國食品藥品管理局頒發的快速通道稱號,用於治療該患者群體,而Genprex的SCLC計劃已獲得美國食品藥品管理局孤兒藥稱號。Genprex的糖尿病基因治療方法由一種新的輸液過程組成,該過程使用AAV載體將Pdx1和mafA基因直接輸送到胰腺。在 1 型糖尿病模型中,GPX-002 將胰腺中的 α 細胞轉化爲功能性 β 樣細胞,這些細胞可以產生胰島素,但可能與 β 細胞截然不同,足以逃避人體的免疫系統。採用類似的方法,用於不起自身免疫作用的 2 型糖尿病的 GPX-002 被認爲可以恢復活力並補充耗盡的 β 細胞。
Interested investors and shareholders are encouraged to sign up for press releases and industry updates by visiting the Company Website, registering for Email Alerts and by following Genprex on Twitter, Facebook and LinkedIn.
Cautionary Language Concerning Forward-Looking Statements
關於前瞻性陳述的警示性語言
Statements contained in this press release regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are made on the basis of the current beliefs, expectations and assumptions of management, are not guarantees of performance and are subject to significant risks and uncertainty. These forward-looking statements should, therefore, be considered in light of various important factors, including those set forth in Genprex's reports that it files from time to time with the Securities and Exchange Commission and which you should review, including those statements under "Item 1A – Risk Factors" in Genprex's Annual Report on Form 10-K for the year ended December 31, 2023.
本新聞稿中有關非歷史事實事項的陳述是1995年《私人證券訴訟改革法》所指的 “前瞻性陳述”。這些前瞻性陳述是根據管理層當前的信念、預期和假設做出的,不能保證業績,並且存在重大風險和不確定性。因此,應根據各種重要因素來考慮這些前瞻性陳述,包括Genprex不時向美國證券交易委員會提交併應審查的報告中列出的那些因素,包括Genprex截至2023年12月31日止年度的10-K表年度報告中 “第1A項——風險因素” 下的陳述。
Because forward-looking statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements regarding: Genprex's ability to advance the clinical development, manufacturing and commercialization of its product candidates in accordance with projected timelines and specifications; the timing and success of Genprex's clinical trials and regulatory approvals; the effect of Genprex's product candidates, alone and in combination with other therapies, on cancer and diabetes; Genprex's future growth and financial status, including Genprex's ability to maintain compliance with the continued listing requirements of The Nasdaq Capital Market and to continue as a going concern and to obtain capital to meet its long-term liquidity needs on acceptable terms, or at all; Genprex's commercial and strategic partnerships, including those with its third party vendors, suppliers and manufacturers and their ability to successfully perform and scale up the manufacture of its product candidates; and Genprex's intellectual property and licenses.
由於前瞻性陳述受風險和不確定性的影響,因此實際結果可能與此類前瞻性陳述所表達或暗示的結果存在重大差異。此類聲明包括但不限於以下方面的聲明:Genprex根據預計的時間表和規格推進其候選產品的臨床開發、製造和商業化的能力;Genprex臨床試驗和監管批准的時機和成功;Genprex的候選產品,單獨或與其他療法聯合使用對癌症和糖尿病的影響;Genprex的未來增長和財務狀況,包括Genprex的保持對持續上市的合規性的能力納斯達克資本市場的要求,繼續作爲持續經營企業,以可接受的條件或完全滿足其長期流動性需求獲得資本;Genprex的商業和戰略伙伴關係,包括與第三方供應商、供應商和製造商的合作伙伴關係,以及他們成功開展和擴大其候選產品製造規模的能力;以及Genprex的知識產權和許可證。
These forward-looking statements should not be relied upon as predictions of future events and Genprex cannot assure you that the events or circumstances discussed or reflected in these statements will be achieved or will occur. If such forward-looking statements prove to be inaccurate, the inaccuracy may be material. You should not regard these statements as a representation or warranty by Genprex or any other person that Genprex will achieve its objectives and plans in any specified timeframe, or at all. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this press release. Genprex disclaims any obligation to publicly update or release any revisions to these forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this press release or to reflect the occurrence of unanticipated events, except as required by law.
不應將這些前瞻性陳述作爲對未來事件的預測,Genprex無法向您保證這些陳述中討論或反映的事件或情況將會實現或將會發生。如果事實證明此類前瞻性陳述不準確,則不準確性可能是重大的。您不應將這些聲明視爲Genprex或任何其他人對Genprex將在任何特定時間範圍內實現其目標和計劃的陳述或保證,或者根本不是。提醒您不要過分依賴這些前瞻性陳述,這些陳述僅代表截至本新聞稿發佈之日。除非法律要求,否則Genprex不承擔在本新聞稿發佈之日後公開更新或發佈對這些前瞻性陳述的任何修訂的義務,無論是由於新信息、未來事件還是其他原因造成的,或者是爲了反映意外事件的發生。
Genprex, Inc.
Genprex, Inc.
(877) 774-GNPX (4679)
(877) 774-GNPX (4679)
GNPX Investor Relations
GNPX 投資者關係
GNPX Media Contact
GNPX 媒體聯繫人
Kalyn Dabbs
Kalyn Dabbs
譯文內容由第三人軟體翻譯。
