Niuniu knocked on the blackboard:

1) the first wave of baby boomers after the founding of New China will reach the age of 60 one after another in 2022. In order to reduce the burden of domestic health insurance, China continues to introduce relevant policies such as collection and negotiation of health insurance, but this has changed the original life cycle of drugs, and the way for domestic pharmaceutical companies to survive in the future: innovating and opening up overseas markets.
2) it is recommended to pay attention to PD-1 and ADC drugs in the field of innovative drugs in 2021. In the field of PD-1, Hengrui Medicine has the most approved indications for domestic PD-1. INNOVENT BIO has better data related to lung cancer, and has authorized Eli Lilly and Co to carry out global commercialization; ADC is known as "biological missile", and Rongchang is the domestic ADC with the fastest progress, many indications and great market potential in the future. Wuxi Biologics is the leader of domestic CXO and has one-stop service for ADC drugs in the world.
Under the epidemic situation in 2020, the pharmaceutical industry has obvious comparative advantages, and its financial performance and market income are in the forefront. As a rigid demand in domestic demand, medicine is "the industry under the spotlight". In 2021, we propose to pay attention to the post-harvest "medical devices", "medical services + self-expense consumption", "innovative drug industry chain" and other races.
2018 can be used as the first year of domestic innovative drugs in China.With the formulation of a series of laws and regulations, such as health insurance collection and procurement, the price of low-end generic drugs has fallen sharply, forcing enterprises to abandon the traditional low-end imitation route and embark on the road of high-end generic drug research and development. Health insurance continues to "vacate cages for birds", and the payment structure tends to be innovative drugs. China's domestic innovative drugs began to be approved and listed in 2018. From the point of view of time, 2018 can be used as the first year of domestic innovative drugs.
"living long" is the single biggest risk of cancer.At present, China has the highest annual incidence of malignant tumors in the world, and the incidence and mortality will further increase rapidly. According to a study in the global authority Science magazine, age is the biggest risk factor for cancer. As we can see from the chart below, the longer the average life expectancy, the higher the incidence of cancer.
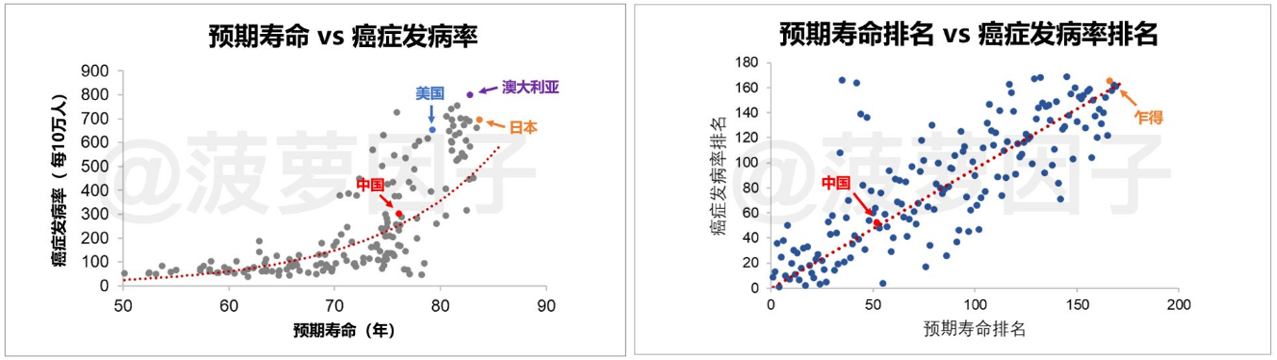
Data source: pineapple factor official account, Futu Securities collation
The period from 1962 to 1972 was the best part of the first wave of babies born after the founding of the people's Republic of China. From 2022, these people will turn 60 one after another. In the future, the number of people suffering from cancer in our country will further increase, and at the same time, it will greatly increase the burden of our medical insurance fund. In order to reduce the burden of health insurance, relevant policies such as collection and health insurance catalogue have been introduced continuously, which has changed the life cycle of drugs.The way for domestic pharmaceutical companies to survive in the future is to innovate and open up overseas markets.

Data source: CITIC Research Institute, Futu Securities arrangement
The domestic tumor drug market is still dominated by traditional chemotherapy drugs, and there is great potential for innovative drugs in the future.Among the ten best-selling antineoplastic drugs in the world in 2019, biological innovative drugs account for 60%, while there are only three biological drugs in China, and the others are chemical drugs, it can be seen that there is great potential for biological innovative drugs in China in the future.
(note: traditional chemotherapy is highly lethal and has great side effects, which can be said to kill 1000 enemies and 800 self-damage.)

In antineoplastic drugs, drugs are mainly divided into chemical drugs and biological drugs. The research and development of biological drugs and pharmaceuticals are difficult, with good efficacy and low side effects, which is the main battlefield of innovative drug research and development in the future. After long-term development, McAbs have entered the stage of technological maturity. In the future, antibody coupling drugs (ADC) and bispecific antibodies will be the core R & D drugs of the company in the short and medium term.

Data source: online public information, Futu Securities collation
Investment opportunities for domestic innovative drugs
1) domestic PD-1 McAb competition will be launched in lung cancer and international market.It is recommended to pay attention to Hengrui Medicine and INNOVENT BIO
In theory, PD-1 inhibitors can treat all cancers.PD-1 inhibitors bind directly to human T cells, so cancer cells cannot bind to human T cells and activate the body's immune system to destroy cancer cells. Therefore, theoretically speaking, PD-1 inhibitors are suitable for many cancers, such as lung cancer, liver cancer, gastric cancer and so on. In addition, PD-1 inhibitors do not produce drug resistance and can be used for a long time.

Photo: Emory University Cancer Research Institute
Domestic PD-1/PD-L1 competition has become the Red Sea, it is recommended to pay attention to the four domestic PD-1 leading enterprises.At present, the four domestic PD-1 products are listed in the national health insurance catalogue. It is estimated that after the four domestic PD-1 products enter the health insurance, the price of the patient's out-of-pocket part is about 15000 / year-30, 000 / year, and then the enterprise advantage approved by PD-1 will gradually weaken.

In the short termHengrui Pharmaceutical accounts for 40% of the domestic market share and has become the "king" of domestic PD-1.No matter from the point of view of price, academic promotion and sales, Hengrui Medicine is in a leading position. Hengrui is the third PD-1 in China, but it has been approved for most indications such as lung cancer, liver cancer, esophageal cancer and so on. Under the premise that Heng Rui PD-1 did not enter the medical insurance, its sales in the first half of 2020 exceeded 2 billion yuan, accounting for 20% of the company's total revenue and 40% of the domestic PD-1 market share, making it the "king" of domestic PD-1.
The main battlefield of PD-1 in the future will be launched in the field of lung cancer and the international market.According to Keytruda (Merck & Co Inc), the king of PD-1 in the world, 22 cancers can be treated, but more than 60% of the income comes from lung cancer, so the main battlefield of domestic PD-1 in the future will be in the field of lung cancer.
Domestic PD-1 has the same data as Merck & Co Inc's PD-1 (Pablizumab) in the treatment of lung cancer, especiallyThe data of INNOVENT BIO's PD-1 lung cancer is better than that of Merck & Co Inc's original drug PD-1, and BeiGene, Ltd. 's ORR data is the best.The indication of Hengrui PD-1 lung cancer has been approved in 2020, and the lung cancer of Cinda, Baiji and Junshi will be officially approved and listed in 2021, which is about to start the PD-1 war.

In 2021, it is recommended to give priority to Heng Rui and Xinda, followed by BeiGene, Ltd. and Junshi Bio.According to incomplete statistics, the sales of Hengrui PD-12020 has exceeded 2 billion in the first half of the year, and it is estimated that the annual sales in 2020 will exceed 5 billion yuan. INNOVENT BIO expects his annual income to exceed 2 billion yuan in 2020. According to the conservative forecast of the agency, Heng Rui's PD-1 income may exceed 80-10 billion yuan in 2021; with the approval of INNOVENT BIO's lung cancer, Xinda's PD-1 income is expected to be 40-5 billion yuan in 2021; BeiGene, Ltd. and Junshi's PD-1 will be listed in the health insurance catalogue in December 2020, and their sales will also be released in 2021.

2) ADC drugs are known as"biological missile",Rongchang biology is making the fastest progress.
ADC drugs are known as "biological missiles" and belong to precision magnified chemotherapy.ADC drugs = Monoclonal Antibodies + potent Cytotoxic drugs + Connectors. ADC drugs connect the bioactive small molecular drugs to the monoclonal antibody through a chemical link, and the monoclonal antibody is used as a carrier to target the small molecular drugs into the target cells, thus improving the targeting of tumor drugs and reducing side effects.

Photo source: leopard Technology
The first year of commercialization of ADC drugs in China will begin in 2021. It is recommended to pay attention to Rongchang organism, which is making the fastest progress and has the most indications.With the continuous expansion of targets, ADC drugs are expected to become the next blue ocean market for oncology drugs. At present, Rongchang's HER2 target ADC drug is in the stage of application for listing, which is the fastest in China and is expected to be approved for market in the first quarter of 2021. The second clinical progress of domestic ADC is Baiotai, which is currently in clinical Ⅲ phase. Baiotai disclosed few targets and indications, and its market space potential is not as good as that of Rongchang Bio.

Patent platform is the core key point of ADCs pipeline expansion, it is recommended to pay attention to Rongchang biology, Wuxi Biologics.Rongchang has established a leading ADC production and R & D platform, screened a variety of coupling drugs, connectors and payload components in drug candidates, and developed a Thiel-Bridge technology platform, which will develop more ADC target drugs in the future.
Wuxi Biologics is the leader of CXO in China, and has only a handful of enterprises in the world that provide one-stop service from antibody and antibody coupling drug solution to preparation. WuXiDAR4TM technology platform has the ability to handle OEB, the annual production capacity of ADC coupling solution can reach 100kg, and the annual production capacity of filling freeze-dried preparation can reach 500000 bottles.

Summary
1) the first wave of baby boomers after the founding of New China will reach the age of 60 one after another in 2022. In order to reduce the burden of domestic health insurance, China continues to introduce relevant policies such as collection and negotiation of health insurance, but this has also changed the original life cycle of drugs and the way for domestic pharmaceutical companies to survive in the future: innovating and opening up overseas markets.
2) attention should be paid to PD-1 and ADC drugs in 2021. In the field of PD-1, Hengrui Medicine currently has the most approved indications in domestic PD-1. INNOVENT BIO's lung cancer-related data is better, and has been authorized to Eli Lilly and Co for global commercialization; in the field of ADC drugs, Rongchang Biological is the domestic ADC with the fastest progress, many indications and great market potential in the future. Wuxi Biologics is the leader of domestic CXO and has one-stop service for ADC drugs in the world.
Edit / elisa
