Source: brokerage China
Author: promise
On the 16th, vaccine research and development institutions in China and the United States announced new progress, which further pushed up the expectation that vaccine concept stocks would continue to be active in A shares and US stocks.
On June 16, the blind review and periodic uncovering blind meeting of novel coronavirus inactivated vaccine Ⅰ / Ⅱ, developed by Wuhan Biological products Research Institute of Chinese Medicine, was held simultaneously in Beijing and Henan. Experts from the vaccine special class of the Joint Prevention and Control Mechanism of the State Council and the Biotechnology Development Center of the Ministry of Science and Technology attended. On the same day, CEO Bansel of Moderna Inc, a biotech company and a US anti-epidemic concept stock, said that if all went well, the final phase of clinical trials of its COVID-19 vaccine was expected to begin in July.
Affected by the progress of vaccine development, vaccine concept stocks continue to strengthen in A shares, US stocks and Hong Kong stocks, because the US vaccine concept stocks directly act as the main body of research and development, and the stock price performance is particularly fierce. The share price of Inovio, which Bill Gates invested in, has risen as much as five times this year, while the share price of Moderna Inc has risen more than twice this year.
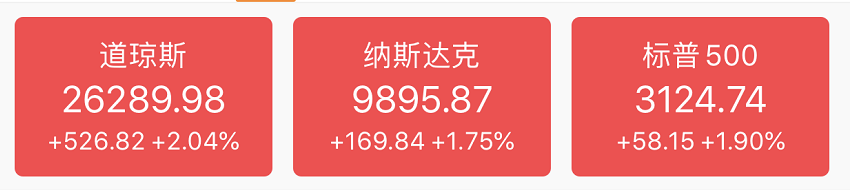
Or as a result, u.s. stocks soared again, with the Dow up more than 700 points in intraday trading, the s & p 500 up more than 2.6%, and the Nasdaq up more than 2%.By the close, the Dow was up 2.04%, the S & P 500 was up 1.9%, and the NASDAQ was up 1.75%.
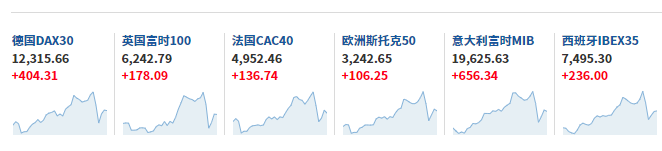
European stocks also rallied, with the European Stoxx 50 index up 3.33%.
A meeting on the clinical blindness of vaccine in China has been held.
On June 16, the blind review and periodic uncovering blind meeting of novel coronavirus inactivated vaccine Ⅰ / Ⅱ, developed by Wuhan Biological products Research Institute of Chinese Medicine, was held simultaneously in Beijing and Henan. Experts from the vaccine special class of the Joint Prevention and Control Mechanism of the State Council and the Biotechnology Development Center of the Ministry of Science and Technology attended.
The results showed that the vaccine was safe after vaccination, and there was no serious adverse reaction. After vaccination with different procedures and doses, people in the vaccine group produced high titers of antibodies, and the positive conversion rate of neutralizing antibodies reached 100% after two doses were inoculated on the 28th day.
According to media reports, the purpose of this study was to evaluate the safety and immunogenicity of the new crown inactivated vaccine in healthy subjects aged 18-59 years old, according to different procedures of low, medium and high doses and 0meme 14, 0prime21 and 0mem28, focusing on the changes of cellular immunity after vaccination, and exploring the changing trend of immune procedure, immune dose, safety, immunogenicity and antibody level in vivo. Up to now, there are 1120 subjects in the Ⅰ / Ⅱ phase clinical study, all of whom have completed two injections.
The clinical trial scheme has been carefully designed, the process of uncovering blindness strictly follows science and rigor, and the results are encouraging, safe and effective after vaccination. All the subjects in the vaccination group produced high titers of antibodies. In the 18-59-year-old group, the positive seroconversion rate of neutralizing antibodies was 97.6% after 14 days and 21 days of vaccination, and 100% of the neutralizing antibodies were inoculated according to the 28-day procedure.
Liu Jingzhen, chairman and secretary of the party committee of China Pharmaceutical Group Co., Ltd. (Sinopharmaceutical Group), disclosed in Beijing on May 29th that Wuhan Biological products Research Institute of Sinopharmaceutical Group and Beijing Biological products Research Institute received clinical approval for new crown inactivated vaccine on April 12th and April 27th respectively, which are now in phase Ⅱ clinical practice, leading the world. At present, the clinical data of more than 2000 cases show that the safety and effectiveness of the vaccine have been fully verified, and there is no obvious adverse reaction.
The National Pharmaceutical Group launched voluntary vaccination at the end of March. The human predictive test of 180 volunteers showed that the antibody of the subjects had completely reached the level of resistance to novel coronavirus, and the protection rate was 100%.
The fund pays close attention to vaccine development.
A-share funds have been paying close attention to the beneficiaries of COVID-19 vaccine research and development, but there are no listed companies as the main body of R & D in the A-share market.
However, this does not stop the speculation of funds, as biomedical stocks have become the mainstream allocation of institutional investors as a result of the epidemic, and the overall performance of pharmaceutical stocks is stable, so even if you buy the wrong stocks, it may not be equal to investment failure.
Under the background that there is no fear of buying mistakes, Sino Biopharmaceutical, which once focused on the listing of Hong Kong stocks with speculative funds.
Sino Biopharmaceutical has been getting a lot of financial attention since the development of COVID-19 vaccine was reported by Sinopharm China Biological Company in April, even though Sino Biopharmaceutical is not the same thing as Sinopharm's China Biotechnology Co., Ltd.
Sino Biopharmaceutical has become extremely active since April because of its similar name, and Sino Biopharmaceutical's shares in Hong Kong rose nearly 50 per cent in the two months to the close of trading on June 16.
Some market participants pointed out that hot money is really not afraid to buy the wrong pharmaceutical stocks, so this kind of varieties are continuing to be sought after by funds, whether or not they are related to COVID-19. In addition, considering that the market value of Sino Biopharmaceutical's stock is more than 170 billion Hong Kong dollars, such a large market capitalization also means that Sino Biopharmaceutical has good fundamentals, even if it "buys the wrong stock" by hyping the companies of Sinopharmaceutical Group. The latter's fundamentals will also ensure the stability of the investment.
Tianfeng Securities recently recommended Sino Biopharmaceutical Company, which recommended Hong Kong stocks, and pointed out that the company has rich reserves of pipeline products and obvious advantages in sales channels, although some varieties may be affected by volume procurement in the short term, but the company's long-term development is worth looking forward to. The company is in the stage of simultaneous development of generics and innovative drugs, considering the production and approval expectations of the blockbuster product PD-1 monoclonal antibody and recombinant clotting factor Ⅷ, the company is expected to usher in an upward trend after digesting the impact of collection. It is estimated that the company's net profit from 2020 to 2022 will be 3.489 billion yuan, 4.11 billion yuan and 4.816 billion yuan respectively. With reference to the industry, the Hong Kong stock pharmaceutical leader will be given 50 times PE in 2020, corresponding to a target price of HK $15.06, which will be upgraded to "buy" rating.
In addition to Sino Biopharmaceutical, which is listed on Hong Kong stocks, Sinopharm Group, listed in Hong Kong shares, A-share listed Sinopharm shares, and Tiantan Biology are also hyped as beneficiaries of vaccine development by Sinopharm Group, and the latter three companies have a close relationship with Sinopharm Group. For example, A-share listed Tiantan Biology was expected by brokers as early as a decade ago to become an integration platform for Sinopharmaceutical Group's biological assets.
Not only does hot money pay attention to this ethereal concept, but even foreign institutions seem to be not immune from vulgarity. Just a few days before the COVID-19 vaccine blindness meeting of the National Pharmaceutical Group, Xiaomo also made a move in Hong Kong stocks. According to the latest information from the Hong Kong Stock Exchange, on June 4, Xiaomo increased its stake in Sinopharm Group (01099) about 4.426 million shares at a price of HK $20 per share, with a total amount of about HK $88.5204 million. After the increase, the latest number of shares is about 95.2553 million shares, and the latest shareholding ratio is 7.09%.
On the day before the blind meeting of the clinical trial of the vaccine, Guotai Junan Securities released a research report on June 15, saying that the overweight rating of Sinopharm Group was maintained, and the stock target price corresponds to 11.6 times 2020 price-to-earnings ratio and 10.1 times 2021 price-to-earnings ratio. That means Sinopharm Group's share price has 43.6% room to rise.
The strongest vaccine concept stocks in US stocks soared fivefold in half a year.
It is worth mentioning that on the same day of the China National Pharmaceutical Group's anti-epidemic clinical exposure meeting, CEO Bansel of Moderna Inc, a biotechnology company and the US anti-epidemic concept unit, said that if all goes well, its COVID-19 vaccine is expected to start the final phase of clinical trials in July, and data on the effectiveness of its novel coronavirus vaccine could be obtained before Thanksgiving at the earliest and put on the market in early 2021.
Chinese reporters at the brokerage have noticed that Moderna Inc's share price has more than tripled so far this year, becoming a bull stock in the US stock market in 2020. The mRNA vaccine, which the company has partnered with the US National Institute of Allergy and Infectious Diseases (NIAID), is currently one of the fastest vaccines in the world.

The company's novel coronavirus vaccine is currently in the second phase of trials, the final phase of which will begin next month in 30, 000 people. At best, Bansel says, "We may get the validity data before Thanksgiving." This is the best timeline. "
It is reported that the US Food and Drug Administration (FDA) will decide whether to approve the vaccine. "they may decide to approve emergency use for high-risk people," Bansel said. "it will be evaluated more carefully before deciding whether to apply it to a wider population.
In addition, Inovio, Pfizer Inc and AstraZeneca PLC are all competing fiercely for vaccine research and development, which has become one of the biggest highlights of the US stock market this year, although they all have great uncertainty.
In terms of stock price and market capitalization, Moderna Inc seems to be far better than Inovio. But Inovio's share price has risen much more than all vaccine concept stocks, and market analysts believe that the difference is likely to be due to the fact that Inovio is the vaccine developer chosen by Bill Gates.
According to public information, Inovio was funded by Bill Gates and his wife's Melinda Foundation Bill & Melinda Gates Foundation. The foundation also announced on February 5 that it would invest US $9 million in Inovio to be distributed by the Alliance for Innovation in epidemic Preparedness (Coalition for Epidemic Alliance for disaster Preparedness Innovation) to speed up the development and testing of the COVID-19 vaccine.
How much have Inovio's shares risen so far this year? It has fully tripled, while the highest share price has risen more than fivefold.

However, analysts in U. S. stocks have pointed out that the revenue expectations of these companies no longer match their valuations. Johnson & Johnson's chief scientific officer has estimated that, based on production, the cost of his COVID-19 vaccine could be as low as "$10 or 10 euros per dose". Therefore, even if the vaccines of these US companies enter the market, fierce competition and government pressure will weaken their pricing power and erode their profitability. if you add the huge investment in research and development and the possibility of the failure of subsequent vaccine technology, it further increases the valuation risk of these concept stocks.
Edit / Phoebe
