Editor / Futu Information Bob
Futu News on February 27th, Moderna Inc, an American biotechnology company, announced its latest financial results for the fourth quarter of fiscal year 2019. Although it continued to lose money, its share price still rose nearly 23% at the close of trading yesterday. As of press time, Moderna Inc rose 21.57% to $35.45 before trading today, and the pre-market offer once again set a new record.
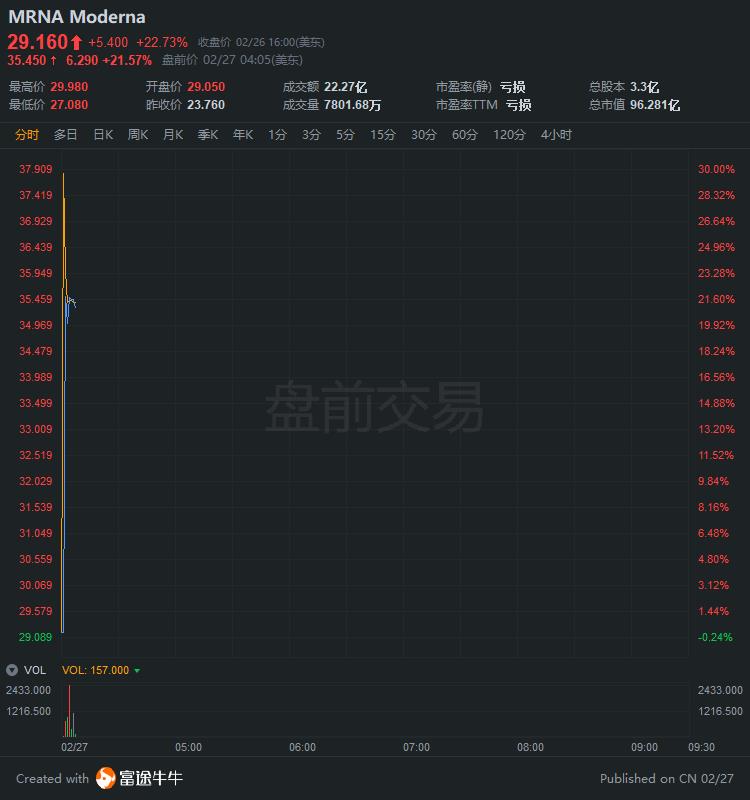
At the same time, before trading today,$American concept of epidemic Resistance (BK2440.US) $Stocks generally soared, NovaVax once rose more than 26% at one time, COMI DiaGNOSTICS I once rose more than 23%, and at one point, alpha Pro technology rose more than 29%.
According to the financial report, Moderna Inc posted a net loss of $124 million in the fourth quarter of 2019 and a loss of $514 million for the whole of 2019, an increase of 33 per cent year-on-year.
Fourth-quarter revenue was $14.1 million, falling short of analysts' expectations and $60.2 million for all of 2019, down 55 per cent from a year earlier.
The company spent $118.7 million on research and development in the fourth quarter, down 21% from a year earlier. Expenditure for the whole of 2019 was $496.3 million, a slight increase over the previous year.

In addition, Moderna Inc founder Stephen Banzer expressed confidence in the mRNA platform used by the company to develop the vaccine on the earnings call. An analyst from JPMorgan Chase & Co asked whether Moderna Inc was ready for mass production of the newly developed novel coronavirus vaccine and how big the production capacity was. In response, Banzer said it was too early to predict capacity.
Banzer also said that thanks to the support of investors, Moderna Inc is currently able to invest up to $2 billion to build the world's largest mRNA vaccine company, including an excellent team of scientists and a manufacturing base.
And the company said that novel coronavirus's first batch of preparations have been sent to the US National Institutes of Health (NIH) to begin phase I clinical research, and have completed their own phase I clinical study by February 7, including virus infusion.


