Guangdong Hec Technology Holding's strong advantages in integrated research, production, and sales are the core intrinsic value, and also one of the important reasons why the company's Stocks have been continuously increased by SH->HK Connect funds.
Recently, the domestic influenza virus has surged and made the "hot search". However, a while ago, a certain antiviral drug was reported to be out of stock in several chain pharmacies, which sparked market discussions and garnered high attention from national authorities.
On January 12, the National Health Commission held a press conference in Peking to introduce relevant information about respiratory disease prevention and control. At the meeting, Deputy Director of the Department of Consumer Goods Industry of the Ministry of Industry and Information Technology, Wang Xiaoyang, stated, "The Ministry has organized the production capacity and output information of respiratory disease-related drugs that the public is concerned about, including antipyretic and analgesic drugs, antibiotics, Traditional Chinese Medicine, and small molecule antiviral drugs. Overall, the supply is adequate, and the market is stable."
 Wang Xiaoyang also pointed out: "China is a major pharmaceutical industrial country, with 0.0125 million large-scale industrial enterprises, a strong Industry Chain, large production capacity, and great potential for capacity expansion, possessing rapid mass production capabilities, especially related to respiratory disease drugs, with a wide variety and large capacity, to meet relevant demand. For example, the production capacity of Oseltamivir reaches 1.56 million doses daily, and the stock exceeds 47 million doses, among which the inventory of granules for children exceeds 16 million doses."
Wang Xiaoyang also pointed out: "China is a major pharmaceutical industrial country, with 0.0125 million large-scale industrial enterprises, a strong Industry Chain, large production capacity, and great potential for capacity expansion, possessing rapid mass production capabilities, especially related to respiratory disease drugs, with a wide variety and large capacity, to meet relevant demand. For example, the production capacity of Oseltamivir reaches 1.56 million doses daily, and the stock exceeds 47 million doses, among which the inventory of granules for children exceeds 16 million doses."
In fact, during the peak influenza season, localized drug supply tightness often occurs, which puts high demands on pharmaceutical supply chain companies. Therefore, only companies that possess market voice and can flexibly implement reasonable production and sales arrangements throughout the drug production and sales cycle according to market dynamics can stand out in the market and continuously consolidate their competitive edge.
This may be one of the reasons why SH->HK Connect funds have continuously increased their investment in HEC CJ PHARM (01558) recently.
The demand for anti-influenza drugs has significantly increased, and southbound funds have continuously increased their positions.
Since November 2024, the positive rate of influenza virus testing in our country has shown a continuous upward trend. On January 9, the China Centers for Disease Control and Prevention released the national sentinel monitoring data for acute respiratory infectious diseases for week 1 of 2025.
The results indicate that the overall trend of acute respiratory infectious diseases continues to rise, with differences in infection trends caused by various pathogens. Influenza is still in the seasonal epidemic period, and the positive rate of influenza virus has slowed down. Among them, the positive rate of influenza virus in flu-like cases in nationwide emergency departments has increased by 3.8% compared to last week, reaching 35.5%.
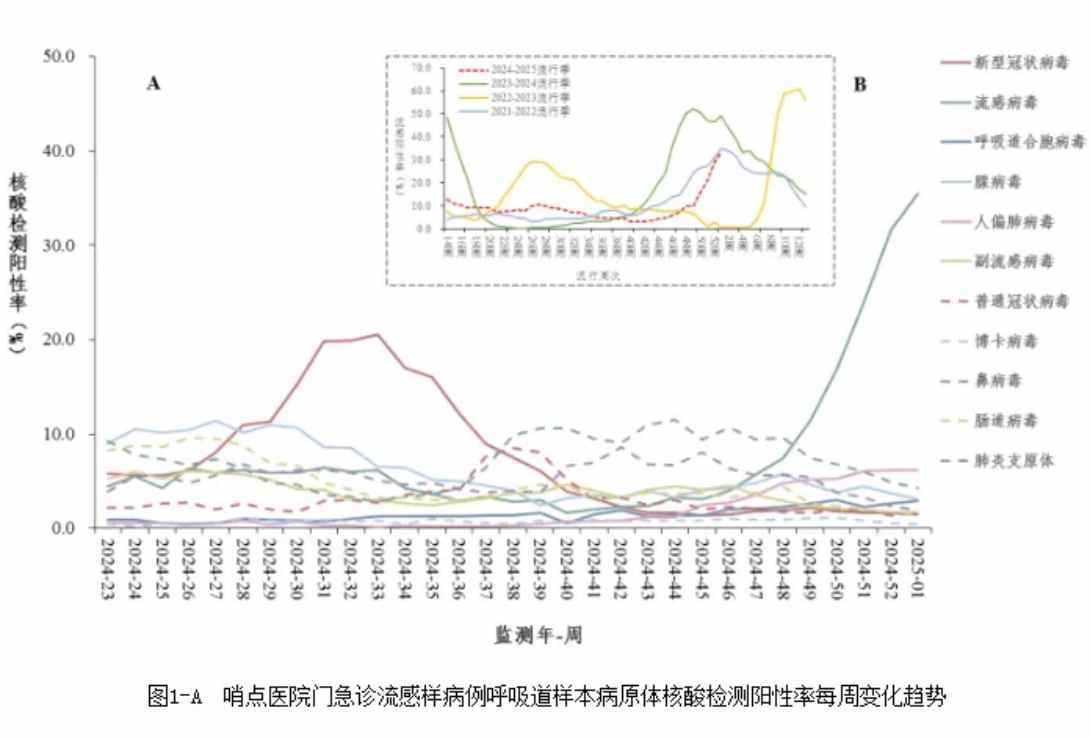
The surge in demand for antiviral drugs against influenza has driven the rapid response of the secondary market in both A and H shares. On January 6, the A-share pharmaceutical sector started a round of sector rotation speculation, with influenza concept stocks rising sharply. More than 10 stocks, including Shandong Lukang Pharmaceutical, Guangdong Zhongsheng Pharmaceutical, Harbin Pharmaceutical Group, Henan Taloph Pharmaceutical Stock, and North China Pharmaceutical, reached the daily limit.
Unlike the performance of sector rotation speculation in A-shares, the Hong Kong market seems to prefer a more steady approach. As a heavyweight anti-influenza symbol in the Hong Kong stock market, HEC CJ PHARM has received increased favor from SH->HK Connect funds. According to observations from Zhitong Finance APP, since November of last year, SH->HK Connect funds have accelerated their positions in HEC CJ PHARM, with the shareholding ratio increasing from 14.88% on November 1, 2024, to 21.51% on January 10, 2025, with a total shareholding of 0.141 billion shares, valued at 1.354 billion Hong Kong dollars.
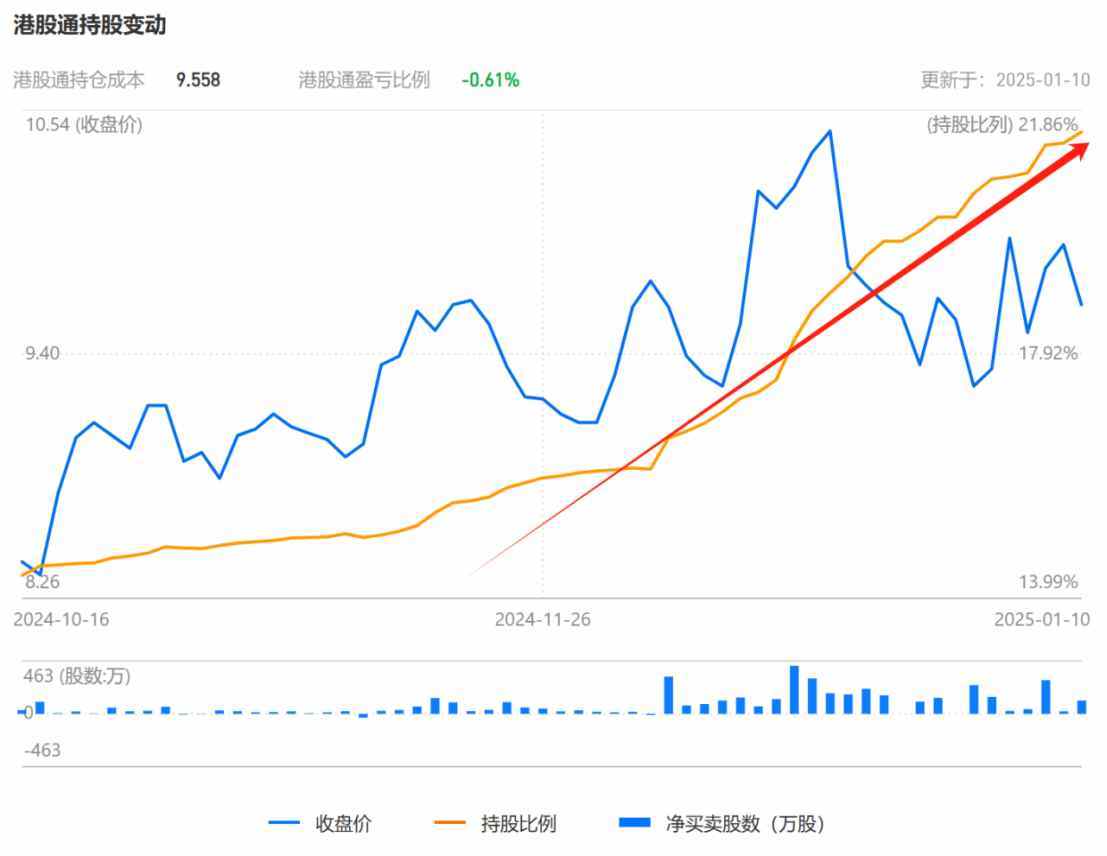
From the trading seats of brokers over the past 60 days, the top three buying brokers for HEC CJ PHARM were SH->HK Connect (Shanghai), SZ->HK Connect, and BNP Paribas, which bought 27.3222 million shares, 19.7514 million shares, and 1.5794 million shares respectively; during the same period, the three main sellers were Bank Of China International, HSBC Hong Kong, and Guoyuan, which sold 9.7086 million shares, 6.945 million shares, and 6.1262 million shares respectively.
In the past 60 days, the net buying amount of SH->HK Connect funds for HEC CJ PHARM accounted for 96.74% of the total net buying amount of the top three brokers, which indirectly reflects the preference of southbound funds for this round of anti-influenza themes, as well as the recognition of the value of HEC CJ PHARM as the leading stock in the domestic anti-influenza concept.

The intrinsic value of the market supply moat.
As a classic drug among anti-influenza medications, Oseltamivir phosphate is known as the "miracle drug for influenza." Oseltamivir phosphate belongs to the class of neuraminidase inhibitors, which can effectively inhibit the activity of neuraminidase in both the A and B strains of the influenza virus, preventing new virus particles from being released from infected cells, thereby disrupting the virus transmission chain and reducing the spread of the influenza virus.
According to the Zhitong Finance APP, the antiviral drug treatment category in the PDB database mainly includes four types of treatment drugs, including Oseltamivir. Statistics show that the total sales of these four drugs in the domestic pharmaceutical terminal market reached 11.405 billion yuan, with Oseltamivir occupying 86.48% of the market share.
Although there are various antiviral drugs available currently, compared to other antiviral drugs against influenza virus, Oseltamivir can be used by pregnant women and children under five years, clinically applied for more than 20 years, ensuring higher safety. A continuous 8-year monitoring result from the "Recommendations for the Prevention and Control of Influenza in Children (2024-2025)" covering nearly 0.14 million cases shows that the overall resistance rate of Oseltamivir has long remained at a low level (0.68%, 944/13980). At the same time, the cost of Oseltamivir is low, can be combined with most drugs on the market, and can also be taken with dairy products and beverages without affecting its efficacy. It comes in various formulations, suitable for a wide range of influenza patients, and is listed as the first choice for influenza in numerous authoritative guidelines both domestically and internationally.
In China, Oseltamivir phosphate is recommended as a first-line antiviral drug for the treatment of influenza in several clinical practice guidelines, such as the "Expert Consensus on Antiviral Treatment of Adult Influenza" and the "Expert Consensus on Diagnosis and Treatment of Influenza in Children (2020 Edition)."
In other words, the market supply situation of Oseltamivir plays a key role in the overall domestic situation against influenza.
However, although influenza regularly occurs every winter and spring, the scale, intensity, and cycle of outbreaks remain unpredictable. When a rapid outbreak occurs, there will be an urgent and large demand for drugs; however, if a large stockpile is held but the influenza epidemic is small and weak, if the inventory time approaches one year, circulation channels may be reluctant to accept it, easily leading to inventory backlogs and waste. It is evident that the instability of drug market demand poses a significant challenge to production enterprises.
Currently, there are more than 60 production enterprises for Oseltamivir phosphate in China, with over 30 actually in production. However, as one of the main producers of Oseltamivir, Guangdong Hec Technology Holding holds a dominant position in the Chinese Oseltamivir market with its Kewai brand (capsules and granules), reaching a market share of about 80%. Behind Kewai's strong ascent is Guangdong Hec Technology Holding's strong integrated research and production sales capability. As an innovative pharmaceutical enterprise, Guangdong Hec Technology Holding not only possesses strong new drug research and development capabilities but also top-notch commercialization and production sales ability.
Guangdong Hec Technology Holding is the largest manufacturer of Oseltamivir in China and the world, with 18 years of industrial development for this product. As a representative drug of the domestic Oseltamivir brand, Guangdong Hec Technology Holding's Oseltamivir capsules "Kexi" was the first to pass the consistency evaluation, gaining widespread recognition from clinicians and becoming well-known among the public.
Compared to the original drugs, Guangdong Hec Technology Holding utilizes a new production technology process, significantly enhancing the production efficiency of Oseltamivir products, with impurity types and quality standards for internal control all higher than those of the original drugs, while continuously engaging in technological innovation to improve product quality based on national standards.
Subsequently, through multiple development initiatives such as technological upgrades and the expansion of industrial scale, the quality of domestic brand products has reached a level comparable to original drugs, production efficiency has greatly increased, and supply capacity is sufficient, ultimately achieving independent domestic supply of key prevention and control drugs without relying on imports.
Currently, the supply of Kexi from Guangdong Hec Technology Holding has established a rapid warning system through a series of interconnected links in market terminals, commercial channels, formulation production, and Active Pharmaceutical Ingredient planning.
More importantly, Guangdong Hec Technology Holding's factory has also established hardware and organizational capabilities for rapid and large-scale production supply in a short time, further improving the entire supply chain. Coupled with their quick monitoring of national influenza information and market response, the supply of key anti-influenza drugs, Kexi, is currently stable and abundant in China.
General Manager Jiang Jun Cai of Guangdong Hec Technology Holding stated in an interview with CCTV Finance Channel: "Currently, the reserves of Active Pharmaceutical Ingredients are sufficient. The granule preparation has a dedicated production workshop with a full capacity of 3.6 billion bags/year; the capsule preparation also has dedicated production during the influenza outbreak season with a full capacity of 3 billion capsules/year. The capacity is sufficient to meet market supply in the event of a large-scale influenza outbreak nationwide. The factory has quickly switched to full production status and has implemented 'three shifts' non-stop production."
Fundamentally, the strong advantage of Guangdong Hec Technology Holding in integrating research, production, and sales is its core intrinsic value and is also one of the important reasons why its stocks have been continuously increased by SH->HK Connect funds.

 另外王孝洋还指出:“我国是医药工业大国,规模以上工业企业有1.25万家,产业链韧性强,生产能力大,扩产潜力也大,具备快速的大规模增产能力,特别是呼吸道疾病相关的药品,种类齐全、产能产量大,可以满足相关的需求。比如,磷酸奥司他韦我们日产能达到了156万人份、库存超过4700万人份,其中儿童用的颗粒剂型库存超过1600万人份。”
另外王孝洋还指出:“我国是医药工业大国,规模以上工业企业有1.25万家,产业链韧性强,生产能力大,扩产潜力也大,具备快速的大规模增产能力,特别是呼吸道疾病相关的药品,种类齐全、产能产量大,可以满足相关的需求。比如,磷酸奥司他韦我们日产能达到了156万人份、库存超过4700万人份,其中儿童用的颗粒剂型库存超过1600万人份。”