In the secondary market, innovative drugs for 2024 are still at a low point. However, no matter how depressed the market is, it will not underestimate companies that release value.
In the secondary market, innovative drugs for 2024 are still at a low point.
According to Changjiang Securities Research Institute, throughout 2024, among the 11 investment themes including AI, AIPC, low-altitude economy, AIGC, Cloud Computing, AI smartphones, Siasun Robot&Automation, CSI Central State-owned Enterprises Composite Index, CSI State-owned Enterprises Composite Index, going overseas, and innovative drugs, innovative drugs are the only theme that has declined overall. However, no matter how depressed the market is, it will not underestimate companies that release value.
 In 2024, 10 innovative pharmaceutical companies had an increase of over 40% within the year, with 3 companies experiencing stock prices that more than doubled.
In 2024, 10 innovative pharmaceutical companies had an increase of over 40% within the year, with 3 companies experiencing stock prices that more than doubled.
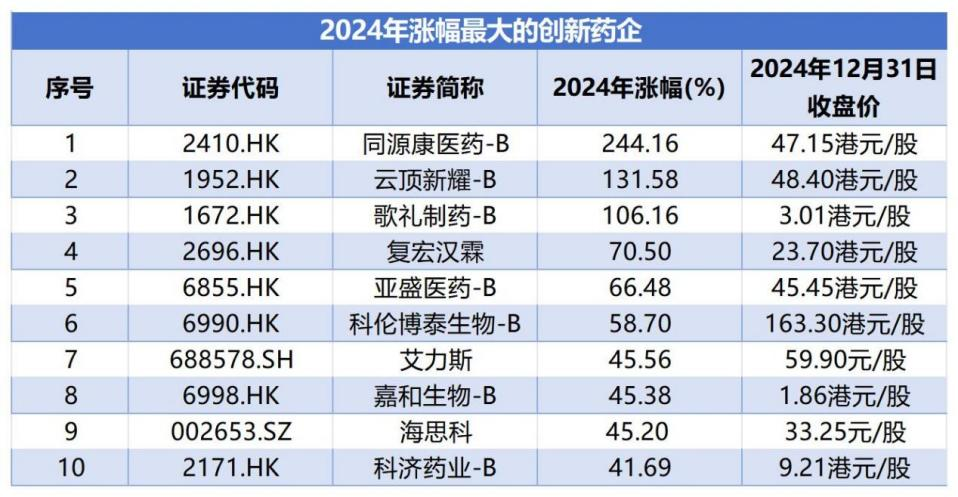
Data source: wind; the increase range is from January 1, 2024, to December 31, 2024.
Among these 10 companies, some reached their peak upon listing, securing wins for two years with a blockbuster product, while others struggled for two years to achieve a turnaround in 2024, elegantly transforming. There are also those who chose to temporarily leave the ‘jianghu’, finding new grounds to realize their value. How to outperform the industry in the changing winds? 10 companies with different profiles and strategies provided their own answers.
Tongyuankang Pharmaceutical (02410): The 'hard currency' of immense wealth.
On August 20 of this year, Tongyuankang Pharmaceutical debuted on the Hong Kong stock market with an issue price of HKD 12.10 per share, subsequently strengthening, with the highest price in December rising from HKD 30.55 to HKD 56.55. In just four months post-listing, the stock price surged by 244%, attracting considerable attention from Hong Kong's biotech sector.
Such a remarkable trend indicates that the market is focused on its core product TY-9591 (Aditinib), which has a significant market for lung cancer and a differentiated advantage, making it very likely to become a major product in the future.
TY-9591 is a deuterated version of Osimertinib developed by Tongyuankang Pharmaceutical, which started the only global 'head-to-head' registration clinical trial in May 2022 for Osimertinib and sole therapy for first-line treatment of EGFR L858R mutation locally advanced or metastatic NSCLC.
By launching the 'head-to-head' claim, the company is emphasizing Osimertinib's leading position in China. However, the NDA for the indications that compete with Osimertinib is expected to be submitted in the second half of 2026. To seize the opportunity, Tongyuankang Pharmaceutical has another breakthrough method: plans to submit an application for conditional listing approval to the National Medical Products Administration in the first quarter of this year, with TY-9591 expected to become the first EGFR inhibitor used for first-line treatment of NSCLC brain metastases.
Brain metastases in NSCLC represent a substantial area in the lung cancer field that has yet to be explored: according to Frost & Sullivan, the incidence of brain metastases in patients with advanced NSCLC can be close to 25% at diagnosis, and approximately 30% to 55% of NSCLC patients will experience brain metastases during treatment. In 2023, it is estimated that there will be 0.1663 million new lung cancer patients with brain metastases in China, expected to reach 0.218 million by 2033.
It is easy to understand why Tongyuankang Pharmaceutical has become a market favorite, with the prospect of a major product launch within a year after its listing.
Ideally, TY-9591 will establish a foothold in the blank market of NSCLC brain metastases, finding a position in the red ocean of NSCLC, and once it wins the 'head-to-head' battle against Osimertinib, it can further expand its indications and gain recognition, leading to its glorious period.
Tongyuankang Pharmaceutical's uniqueness once again proves that a truly differentiated product capable of capturing a large market is valuable currency in any capital environment.
Genting Yongying (01952): Winning the turnaround battle, sprinting towards the 10 billion sales peak.
When listed in 2020, Trodelvy, introduced from Immunomedics, was regarded as the "pillar asset of Genting Yongying's oncology business," making it the most enticing piece of its 20 billion Hong Kong dollar Market Cap. Thus, when Genting Yongying chose to return the rights to Trodelvy in 2022, even holding 3 billion yuan in returns, it faced significant noise from the capital markets, causing the stock price to plummet to its lowest point since listing.
The decision made by Genting Yongying at that time was to "prioritize survival before seeking development," advancing the clinical candidate drugs with FIC or BIC potential in other areas.
Timely cut-loss and withstand doubts, in March 2023, Genting Yongying's pillar asset in the anti-infection business, the world’s first fluorocycline-class antibacterial drug, Icaris, was approved for market launch. In July of the same year, CEO Luo Yongqing directly proposed a goal of "four commercialized products launched in the short term, reaching 10 billion in sales peak."
In 2024, Genting Yongying’s major business pillar assets will concentrate on unleashing commercial potential.
In the first half of the year, the commercialization of Icaris was steadily carried out, with the only approved disease-modifying treatment for IgA nephropathy, Dosingkang, opened its first prescription in May. With these two drugs, Genting Yongying achieved total revenue of 0.302 billion yuan in the first half, with a gross margin of 83% after excluding non-cash items, marking the company's first profitability in the commercial aspect in history.
In the second half of the year, Genting Yongying's pillar asset in the autoimmune business, Iqmod, was also approved for market launch in Macau and the Greater Bay Area, with an expected annual sales target of 0.7 billion yuan. Coupled with Dosingkang now included in the 2024 pharmaceutical catalog, it is expected to ramp up quickly in 2025, establishing a solid foundation for next year’s commercialization expectations.
The remarkable commercial achievements in the first half of the year and continuous progress in the second half garnered positive feedback from the capital markets for Genting Yongying, with stock prices surging from a high of 22.7 Hong Kong dollars per share at the end of August to a high of 52.15 Hong Kong dollars per share by the end of December.
This recognition is not only for the current achievements but also for its discerning "product selection" vision, strategic courage, and strong execution capabilities. Anticipation is high for Geng Tian Xin Yao to achieve its strategic goal of cash break-even by the end of 2025, joining the ranks of Biotech companies that are on the mend, setting a confidence example for China's Innovative Drugs.
Eagle Pharmaceuticals (01672): The First 18A Stock Seeks New Opportunities.
Although it bears the title of "the first 18A stock," Eagle Pharmaceuticals has been silent for a long time. After suffering setbacks in the hepatitis C drug business, it briefly jumped on the COVID-19 product bandwagon. However, after COVID-19 completely cooled down in the first half of 2024, it immediately faced the awkward situation of "not having sales revenue from Lopinavir products," resulting in a net loss of 0.13 billion yuan, an increase of 6.87 times year-on-year.
The next opportunity Eagle Pharmaceuticals seeks is to benchmark against Novo-Nordisk and Eli Lilly and Co's weight loss drugs. A series of clinical developments announced indeed drove the stock price to rise consistently in the last four months of 2024.
On September 17, Eagle Pharmaceuticals announced that its small molecule GLP-1R agonist ASC30 is undergoing two Phase I clinical trials in the USA for monthly subcutaneous injections and daily oral tablet treatments for obesity.
The announcement states that after head-to-head comparisons, ASC30's in vitro efficacy against GLP-1R is 2 to 3 times higher than that of Orforglipron; in intravenous glucose tolerance tests (IVGTT) conducted on non-human primates (NHPs), ASC30 (dosage: 1.5 mg/kg) stimulated more insulin secretion than Orforglipron (dosage: 6 mg/kg), with statistically significant differences. The day after the news release, Eagle Pharmaceuticals opened over 7% higher.
By December 2024, Eagle Pharmaceuticals continued to announce multiple clinical trial updates for its first-in-class candidate drug ASC47 for obesity treatment, especially showing "encouraging efficacy" in studies of ASC47 combined with Semaglutide in diet-induced obesity (DIO) mouse models, further catalyzing the stock price increase.
Regardless of how the market views it, Eagle Pharmaceuticals seems full of confidence in its "self-rescue" efforts. With a series of announcements regarding GLP-1 clinical progress, Eagle Pharmaceuticals has also been actively repurchasing shares, having repurchased nearly 0.2 billion Hong Kong dollars since June 2023, making it the strongest repurchasing effort among Hong Kong's 18A Biotech stocks.
HENLIUS (02696): There are still legends about him in the industry.
Among Hong Kong's innovative drug companies, HENLIUS is perhaps the most troubled by liquidity issues. Compared to Shanghai Allist Pharmaceuticals Co., Ltd. which became immensely wealthy with a me-better product in the A-share market, HENLIUS's core product, the biosimilar Trastuzumab, has achieved even better commercialization results, yet the feedback from the Hong Kong market is increasingly bleak.
Since obtaining approval for market launch in July 2020, Hanquyou achieved revenues of 0.868 billion yuan, 1.695 billion yuan, and 2.644 billion yuan from 2021 to 2023, becoming the only pharmaceutical product among the top innovative drug companies in the Hong Kong stock market that has maintained a growth rate of over 50% after being included in the national medical insurance, especially in 2023, with a strong growth in sales while the domestic market sales expense rate fell to around 30%, which significantly helped HENLIUS to be the first among a host of innovative drug companies to turn a profit that year.
However, in the Hong Kong stock market, HENLIUS's stock price has continuously declined from a peak of 61.50 HKD per share on August 31, 2020, to a high of 17.80 HKD per share by the end of August 2022, subsequently hovering around around ten HKD per share for the next two years.
Neither the company nor the shareholders wish to see such results. On May 22 of this year, FOSUN PHARMA intervened at a price of 24.60 HKD per share, a premium of 52.04% over the previous 30 trading days without disturbance, thus providing HENLIUS a sense of justice.
On December 20, 2024, HENLIUS and FOSUN PHARMA issued a joint announcement stating that the National Development and Reform Commission had approved this transaction on November 21, 2024, and HENLIUS expects to delist from the H-shares by February 7, 2025.
As HENLIUS indicated, due to the H-share trading at relatively low price ranges and subdued trading volumes for most of the past few years, its ability to raise funds from the stock market has been greatly restricted, and the mismatch between stock price and value may also harm its business focus and employee morale.
Merging and delisting will allow HENLIUS to free itself from the quagmire of liquidity, lightening the burden in two ways: reducing the administrative resources needed to maintain its listing status and avoiding price fluctuations and valuation distortions, thus concentrating on resolving key issues related to core business and operation, while seeking new prospects for development.
Ascentage Pharma (02696): The correct use of BD.
A single arrow piercing the clouds, thousands of troops come to meet.
In June 2024, a notice from Ascentage Pharma brought tears of joy to its secondary market shareholders. The long-awaited BD Trade has finally become a reality, giving the outside world stronger expectations for its flagship products' competitiveness in the international market, and directly boosting the stock price of Ascentage Pharma.
On June 14, Ascentage Pharma exclusively licensed the Chinese foreign rights of Olaparib to Takeda Pharmaceutical, and more significantly, Takeda made a $75 million investment acquiring a 7.7% stake in Ascentage Pharma, alongside Ascentage Pharma's upcoming IPO in the USA.
Ascentage Pharma's "triple play" intent is clear: the collaboration with Takeda Pharmaceutical on BD clarifies the market's expectation of its core products' overseas market value, with Takeda being "the most globalized Japanese pharmaceutical company" serving as a second parent company, boosting both Ascentage Pharma and the market's confidence in its true route to internationalization through its IPO in the USA.
Since the mid-term report was released, Ascentage Pharma has shown the strongest performance since its listing, with its stock price rising from a peak of 29.80 HKD/share at the end of June to a highest of 46.75 HKD/share by December 31. Supporting this upward trend is undoubtedly the consensus around the globalization of China’s innovative drugs, enhancing the high expectations for Ascentage Pharma's advancement into the international Biopharma sector.
Kellen Biotechnology (06990): A stable powerhouse, ready for commercialization.
With the immense backing of Merck, a global top pharmaceutical company, the expectations for Kellen Biotechnology have never been about whether it is "good or not," but rather about "how good it can be."
In 2022, before going public, Kolun Botai entered into two licensing agreements with Merck within a year, involving a total of nine ADC assets for cancer treatment, with a total transaction amount reaching 11.8 billion USD, setting records for both the largest biopharmaceutical licensing deal for innovative drug companies in China that year and the largest global biopharmaceutical collaboration, allowing Kolun Botai to establish its reputation in the ADC field and initiate China's ADC outflow trend.
With this backdrop, Kolun Botai's IPO performance can be expected to be impressive. It went public in July 2023, raising a net amount exceeding 1.25 billion HKD, becoming the largest IPO in the biotech sector of the Hong Kong stock market in nearly two years. On the day of listing, Kolun Botai rose 3.14%, closing at 62.5 HKD/share, with a total market cap of 13.489 billion HKD, far surpassing many biotech companies that suffered low stock prices.
Since its listing, Kolun Botai has been bullish. In March 2024, Kolun Botai released its 2023 annual report, with stable revenue from BD and multiple ADCs entering the phase III trial or上市阶段 becoming strong catalysts for its stock price, which surged from a high of 115.5 HKD/share on February 29 to a high of 177.9 HKD/share on March 28, and has since maintained a high level.
On November 27, Kolun Botai reached another crucial milestone: the approval of Lucanto Satuzumab (SKB264/MK-2870, Katali) for listing in China, which is the first domestically produced TROP2 ADC approved for marketing in China and also the first domestically produced ADC to receive full approval for sale, with projected sales of 8 to 1 billion yuan by 2025.
Kolun Botai also successfully garnered the favor of international biopharmaceutical players: as the news broke, JPMorgan gave Kolun Botai a 'Shareholding' rating for the first time, with a target price of 240 HKD, and it is expected that the potential peak sales of Lucanto Satuzumab in China will exceed 8 billion yuan, with peak sales outside China potentially reaching 6 billion USD.
Kolun Botai, which created a myth in BD, is about to validate its commercialization capabilities by 2025.
Shanghai Allist Pharmaceuticals Co., Ltd. (688578.SH): The value of Furmetin is still increasing.
Relying on Furmetin, Shanghai Allist has created a miracle of 'strengthening for two years' amid the capital winter.
Fumitin initially did not stand out. In March 2021, Fumitin became the third approved third-generation EGFR inhibitor in the country; in 2022, Fumitin's sales were far less than the 4.3 billion yuan of Osimertinib and 2.4 billion yuan of Amivantamab, only 0.79 billion yuan.
The "myth" began in 2023. After both first-line and second-line indications were included in medical insurance, Fumitin rapidly gained market volume, achieving operating income of over 2 billion yuan in 2023, with a net income of 0.644 billion yuan. In the first half of 2024, it reached a new high with revenue of 1.576 billion yuan, a year-on-year increase of 110.57%, a net income of 0.656 billion yuan, a year-on-year increase of 214.82%, and EPS of 1.46 yuan, a year-on-year increase of 217.39%.
After the release of the 2023 financial report at the end of April this year, the outstanding financial performance directly drove the share price of Shanghai Allist Pharmaceuticals Co., Ltd. to soar by 42%, reaching nearly 66 yuan per share at its peak.
The only period of declining share prices in the past two years occurred around the release of Shanghai Allist Pharmaceuticals Co., Ltd. 's third quarterly report this year: while facing attacks from several new EGFR inhibitors, the sales expenses of Shanghai Allist Pharmaceuticals Co., Ltd. exceeded 1.2 billion yuan in the first three quarters of 2024, hitting a new high, while the growth rates of revenue and net income declined.
In fact, this decline can also be seen as an adjustment: expectations for Shanghai Allist Pharmaceuticals Co., Ltd. have shifted from the two-year frenzy of Fumitin's sales surge to the mature commercialization capabilities developed through Fumitin.
Over the past four years, Shanghai Allist Pharmaceuticals Co., Ltd. has developed a professional sales team of up to 1,000 people, and despite the continuous growth of sales expenses, the sales expense ratio is trending towards rationality, having dropped to 38.3% in the first three quarters of 2024, making it a leader among innovative pharmaceutical companies.
Competition among EGFR inhibitors is heating up, and an efficient commercialization system will be the decisive point for Biopharma in the lung cancer field. Growth always has a ceiling, and Shanghai Allist Pharmaceuticals Co., Ltd. has already adjusted direction, introducing several products since last year, including the RET inhibitor Pralsetinib from CStone Pharmaceuticals, the KRAS G12C inhibitor Adagrasib from Mirati Therapeutics, and the SHP2 inhibitor JAB-3312. As Shanghai Allist Pharmaceuticals Co., Ltd. moves towards Biopharma, can it create another growth myth? Stay tuned for 2025.
Genscript Biotech (06998): A dignified exit.
This year, the most iconic scene in the Hong Kong stock market for Innovative Drugs is certainly the first reverse merger case of an Innovative Drug company since the implementation of the 18A rule by the Hong Kong Stock Exchange: On October 7, Haisco Pharmaceutical Group announced the merger and acquisition of Immunomedics.
The Capital Markets' attitude towards 'Immunomedics and Haisco' is demonstrated by Haisco Pharmaceutical Group's resumption of trading on the same day, with stock prices rising by 41.82%, closing at 2.34 HKD per share, thus achieving the total annual increase for Haisco Pharmaceutical Group.
Prior to this, Haisco Pharmaceutical Group had been hovering at the low point of '1 HKD stock' for nearly two years. Continuous failures in pipeline progress left this early-stage Biotech missing the bus, with only one biosimilar drug, Infliximab, approved for launch by the first half of 2024, generating only 14.47 million yuan in revenue in the first half; in March, the underwhelming NDA application for its CDK4/6 inhibitor was accepted, which also failed to create much of a stir.
At such a moment, seeking 'external assistance' is the most suitable choice. Haisco Pharmaceutical Group acknowledges that the commercialization of the CDK4/6 inhibitor has reached a critical stage, and the merger aims to leverage Immunomedics' strong marketing network and other capabilities to promote the rapid commercialization of the drug.
A milestone in an era is often marked by the figure that 'departs gracefully' first. However, many new questions remain for Immunomedics and Haisco: Can the R&D capabilities and product pipeline of Haisco Pharmaceutical Group truly complement Immunomedics' commercialization abilities and stable cash flow? Can these two genetically different companies successfully integrate? And perhaps most urgently, how should Immunomedics and Haisco break through the 'tough battle' surrounding the CDK4/6 inhibitor and establish a foothold?
Haisco Pharmaceutical Group (002653.SZ): Innovative Drugs + Going Global, fulfilling the expectations for the transformation of generic drug companies.
Among the top 10 Innovative Drug companies with the largest increases this year, Haisco is the only one transforming from a generic drug company, and besides Shanghai Allist, it is the only company seen as bullish in the past two years, with an increase of about 45% this year.
Just two years ago, in April 2022, after Haisco Pharmaceutical Group released its annual report, its stock price dropped to around 15 yuan per share, hitting a historical low.
Haisco Pharmaceutical Group itself stated that "2021 was a highly challenging year": with the rise of Innovative Drugs, despite its first self-developed Class 1 new drug, the环泊酚 injection, being launched, it has not yet gained traction. Additionally, the significant price reduction of the blockbuster product, injectable macitentan, has led to declines in both revenue and net income, casting a shadow of uncertainty over future development.
At that time, Haisco's primary transformation strategy was confirmed as "laying out the launch of Innovative Drugs with a global perspective," promoting the dual reporting of the环泊酚 injection in both China and the USA, while actively facilitating domestic market development and scaling through healthcare negotiation access.
After two years of transformation, Haisco is set to face two key milestones in 2024, driving a strong rise in its stock price. The first is the announcement of the 2023 annual report at the end of April, returning to a growth rhythm of revenue and net income attributable to the parent company both increasing year-on-year. The环泊酚 injection contributed significantly, not only boosting anesthetic products to become Haisco's "main business," with its share of revenue increasing from 2% to 25%, but also fostering Haisco's transformation into Innovative Drugs.
Second, from August to September, Haisco further disclosed in its semi-annual report that three Innovative Drugs, including the环泊酚 injection, entered the NDA stage in the USA, and later announced the establishment of two wholly-owned subsidiaries overseas, greatly raising expectations for market expansion, resulting in the largest surge in Haisco's stock this year, with a closing price of 36.96 yuan per share on September 30, an increase of over 18%.
Haisco's rise in the past two years proves the positioning of Innovative Drugs as a gold mine; generic drug companies are not inherently weaker than innovative ones, but rather, due to their previous rich operational experience and resources, they have unique opportunities to overtake in twists and turns.
Carlyle Pharmaceuticals (02171): A new story of old CAR-T.
Before its "ascent" in September this year, Carlyle Pharmaceuticals' stock price had briefly fallen below 3 Hong Kong dollars, a historical low, mainly due to the ongoing commercialization difficulties with CAR-T. After the approval of its first commercial product, CAR-T, for market launch in February this year, Carlyle published its semi-annual report at the end of August, reporting revenue of approximately 6 million yuan and a net loss of 0.352 billion yuan.
Compared to the "flying start" of Gilead Sciences, Carlyle Pharmaceuticals appears more like "an old tree blossoming anew," with its stock price skyrocketing over 40% in the last three months, demonstrating clear catalytic events that directly indicate CAR-T's strong attention from the capital market in two directions: one is the allogeneic universal CAR-T, and the other is releasing CAR-T's potential in treating solid tumors.
At the beginning of November, KJ Pharma announced that its products, Sikeza (Zevorocel injection), CT071, and CT0590 data will be presented in poster form at the 66th American Society of Hematology (ASH) annual meeting. A highlight among them is the universal CAR-T cell therapy CT0590.
The summary indicates that CT0590 targets BCMA and NKG2A, and a Phase I study was conducted in patients with relapsed/refractory multiple myeloma (RRMM), enrolling 5 subjects. At a median follow-up of 16.6 months, 3 patients achieved remission, including 2 with stringent complete response (sCR) and 1 with partial response (PR). Among the 2 sCR patients, one RRMM patient's duration of response (DOR) has reached 23 months (sCR is still ongoing), while the other patient's DOR for pPCL is 20 months. In these 2 sCR patients, the peak CAR copy number was greater than 280,000 copies/µg genomic DNA. The safety profile was also quite manageable. Following this news, KJ Pharma's stock surged by 80% over two trading days.
On December 30, KJ Pharma announced that the CT041 injection for treating Claudin18.2 positive advanced gastric/esophagogastric junction adenocarcinoma, which had previously failed at least 2 lines of treatment, met the primary endpoint (PFS) in a key Phase II clinical trial in China. It is expected to submit a new drug application to the NMPA in the first half of 2025, potentially becoming the world's first CAR-T product targeting solid tumors. After the announcement, KJ Pharma's stock price surged over 20% during the trading session.
After the clinical progress drew market attention, the focus for 2025 is on whether KJ Pharma can smoothly become the 'world's first CAR-T targeting solid tumors' and whether it can save the company while providing a bright direction for the CAR-T field.
This article is reprinted from the WeChat public account 'Pharmaceutical Magic Cube,' author: Zhao Jingyi; editor: Yan Wencai from Zhitong Finance.

 2024年,10家创新药企年内涨幅超过40%,3家的股价涨超一倍。
2024年,10家创新药企年内涨幅超过40%,3家的股价涨超一倍。