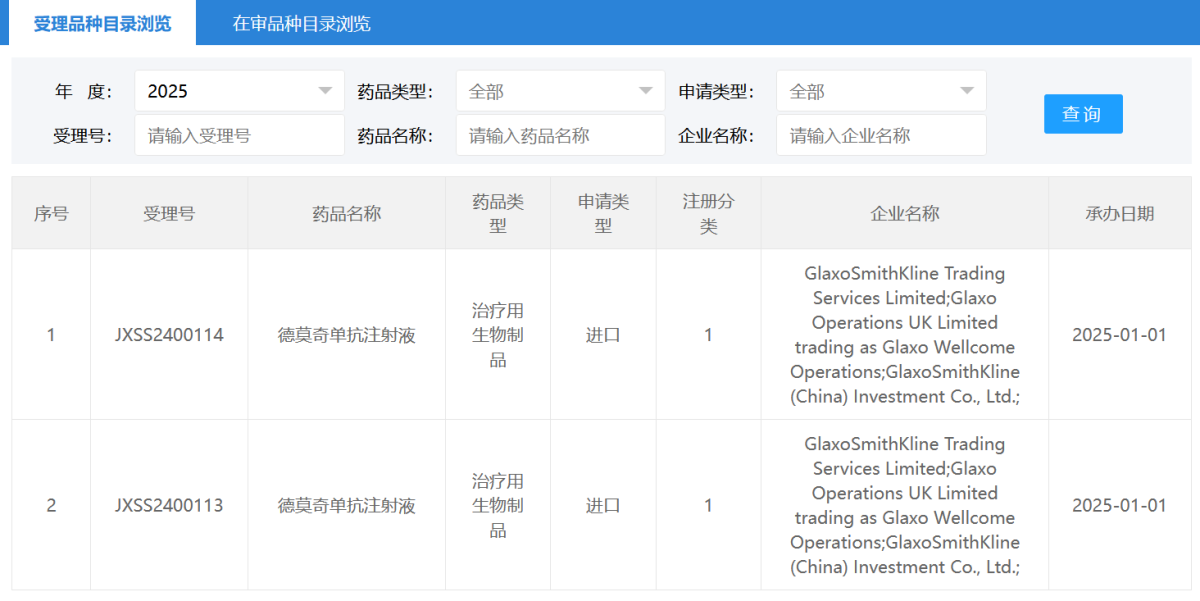
The CDE website shows that GlaxoSmithKline (GSK.US) has applied for the market launch of depemokimab in the country.
According to the Zhitong Finance APP, the CDE website shows that GlaxoSmithKline (GSK.US) has applied for the market launch of depemokimab domestically. Based on the progress of clinical trials, it is inferred that the indication for this application is for severe eosinophilic asthma (Sea).
Public information shows that depemokimab is a new generation anti-interleukin-5 (IL-5) monoclonal antibody developed by GSK, which has a longer half-life, high binding affinity, and high potency, requiring injection only once every six months. Previously, GSK's first-generation IL-5 monoclonal antibody, mepolizumab (trade name: Nucala), was a monthly formulation that was approved for market release in the USA in November 2015. According to GSK's Earnings Reports, global sales of mepolizumab in 2023 were approximately 2 billion dollars.