On December 30, the CDE official website showed that Sanofi (SNY.US) has had its market application for the BTK inhibitor Rilzabrutinib accepted.
According to the Zhitong Finance APP, on December 30th, the CDE official website showed that Sanofi's (SNY.US) BTK inhibitor Rilzabrutinib's marketing application has been accepted. According to the Pharmaceutical Magic database, Sanofi has conducted multiple clinical trials of Rilzabrutinib for the treatment of autoimmune diseases in China, including a Phase III study (CTR20220447) for immune thrombocytopenic purpura (ITP), and a Phase IIb study (CTR20212864) for warm antibody autoimmune hemolytic anemia.
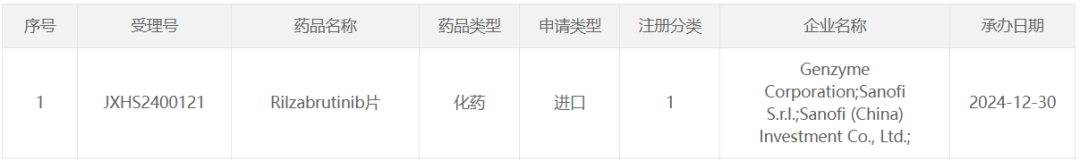
In April 2024, Sanofi announced that the Phase III LUNA 3 study of Rilzabrutinib for adult patients with persistent or chronic immune thrombocytopenic purpura (ITP) achieved its primary endpoint. At that time, Sanofi planned to submit the marketing application for Rilzabrutinib based on this data. If approved, Rilzabrutinib would be the first BTK inhibitor approved for the indication of ITP.
