According to the Hong Kong Stock Exchange's disclosure on December 30, 2023, Yaojie Ankang (Nanjing) Technology Co., Ltd. submitted an application for listing on the Main Board, with CITIC SEC and Huatai International as its joint sponsors.
Zhitong Finance APP learned that, according to the Hong Kong Stock Exchange's disclosure on December 30, Yaotai Ankang (Nanjing) Technology Co., Ltd. (Yaotai Ankang) submitted a listing application to the Main Board of the Hong Kong Stock Exchange, with CITIC SEC and Huatai International as its joint sponsors. It is noted that the company has submitted applications to the Hong Kong Stock Exchange four times.

According to the prospectus, Yaotai Ankang is a biotechnology company that is clinical demand-driven and at the clinical registration stage, focusing on the discovery and development of innovative small molecule therapies for tumors, inflammation, and cardiovascular metabolic diseases. With its fully integrated internal research and development system, the company has established a pipeline of six clinical-stage candidates and one preclinical candidate.
 The company currently has a pipeline of six clinical-stage candidates and one preclinical candidate. The core product Tinengotinib (TT-00420) is a globally innovative drug that is in the registration stage. It is reported that Tinengotinib has the potential to address unmet clinical needs in treating various relapsed or difficult-to-treat, drug-resistant solid tumors (including cholangiocarcinoma, prostate cancer, breast cancer, biliary tract cancers, and pan-FGFR solid tumors). The prospectus also mentions that the company plans to develop commercialization and marketing plans to accompany the anticipated product launch, starting with the commercialization of Tinengotinib for treating cholangiocarcinoma in China. However, there is no guarantee that the company will ultimately succeed in developing and selling its core product or any pipeline products.
The company currently has a pipeline of six clinical-stage candidates and one preclinical candidate. The core product Tinengotinib (TT-00420) is a globally innovative drug that is in the registration stage. It is reported that Tinengotinib has the potential to address unmet clinical needs in treating various relapsed or difficult-to-treat, drug-resistant solid tumors (including cholangiocarcinoma, prostate cancer, breast cancer, biliary tract cancers, and pan-FGFR solid tumors). The prospectus also mentions that the company plans to develop commercialization and marketing plans to accompany the anticipated product launch, starting with the commercialization of Tinengotinib for treating cholangiocarcinoma in China. However, there is no guarantee that the company will ultimately succeed in developing and selling its core product or any pipeline products.
According to Frost & Sullivan data, the number of global cholangiocarcinoma patients increased from approximately 234,900 in 2018 to 280,000 in 2023. About 62% of patients with advanced unresectable or metastatic cholangiocarcinoma receive second-line treatment, while 32% of patients have received at least third-line treatment. Among cholangiocarcinoma patients, 25.2% exhibit FGFR mutations (including fusions and rearrangements, point mutations, and gene amplifications), and 7.4% of patients with partial remission have observed FGFR fusions and rearrangements.
The company currently has no products approved for commercial sale, and has not generated any revenue from product sales. During the reporting period, the company has not achieved profitability and incurred operating losses. Yaotai Ankang's revenue mainly comes from milestone payments related to TT-01025 from LG Chem and external licensing.
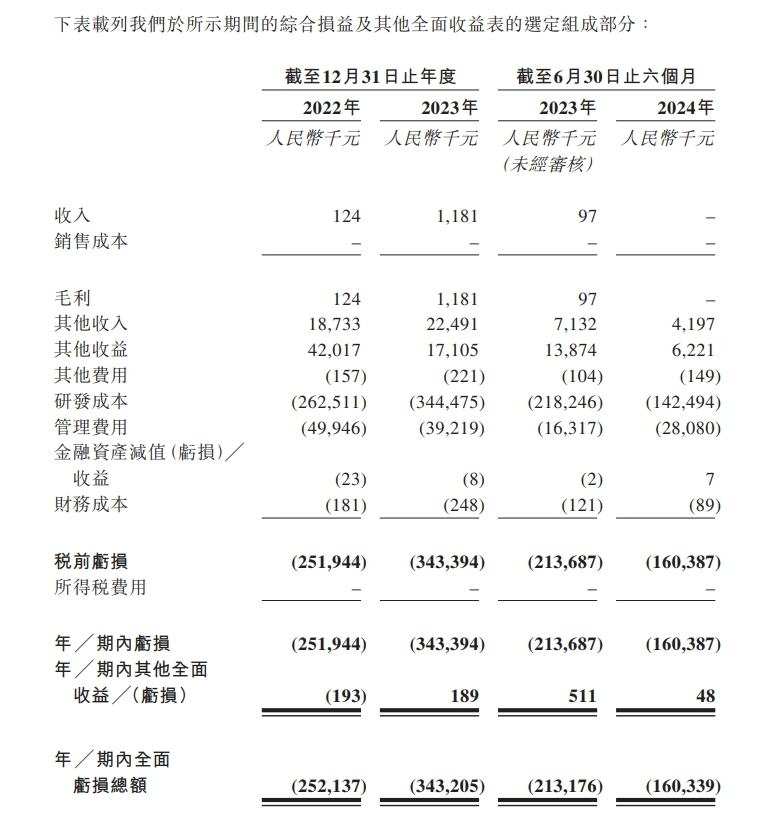
Financial data shows that in 2022, 2023, and from January to June 2024, the revenue of Yaojie Ankang was approximately 0.124 million yuan, 1.181 million yuan, and 0 yuan respectively, while the corresponding annual losses during these periods were approximately 0.252 billion yuan, 0.343 billion yuan, and 0.16 billion yuan.

 公司目前共有六款临床阶段候选产品及一款临床前候选产品的管线。最核心的产品Tinengotinib(TT-00420)为全球首创药物,处于注册阶段。据悉,Tinengotinib有潜力治疗各种复发或难治、耐药实体瘤(包括胆管癌(“胆管癌”)、前列腺癌、乳腺癌、胆道系统癌症(“BTC”)和泛FGFR实体瘤)的未满足的临床需求)。招股书同时提到,公司计划制定商业化及营销计划以配合未来预期的产品上市,首先是在中国进行Tinengotinib用于治疗胆管癌的商业化。但概不保证公司最终将能够成功开发及销售公司的核心产品或任何管线产品。
公司目前共有六款临床阶段候选产品及一款临床前候选产品的管线。最核心的产品Tinengotinib(TT-00420)为全球首创药物,处于注册阶段。据悉,Tinengotinib有潜力治疗各种复发或难治、耐药实体瘤(包括胆管癌(“胆管癌”)、前列腺癌、乳腺癌、胆道系统癌症(“BTC”)和泛FGFR实体瘤)的未满足的临床需求)。招股书同时提到,公司计划制定商业化及营销计划以配合未来预期的产品上市,首先是在中国进行Tinengotinib用于治疗胆管癌的商业化。但概不保证公司最终将能够成功开发及销售公司的核心产品或任何管线产品。