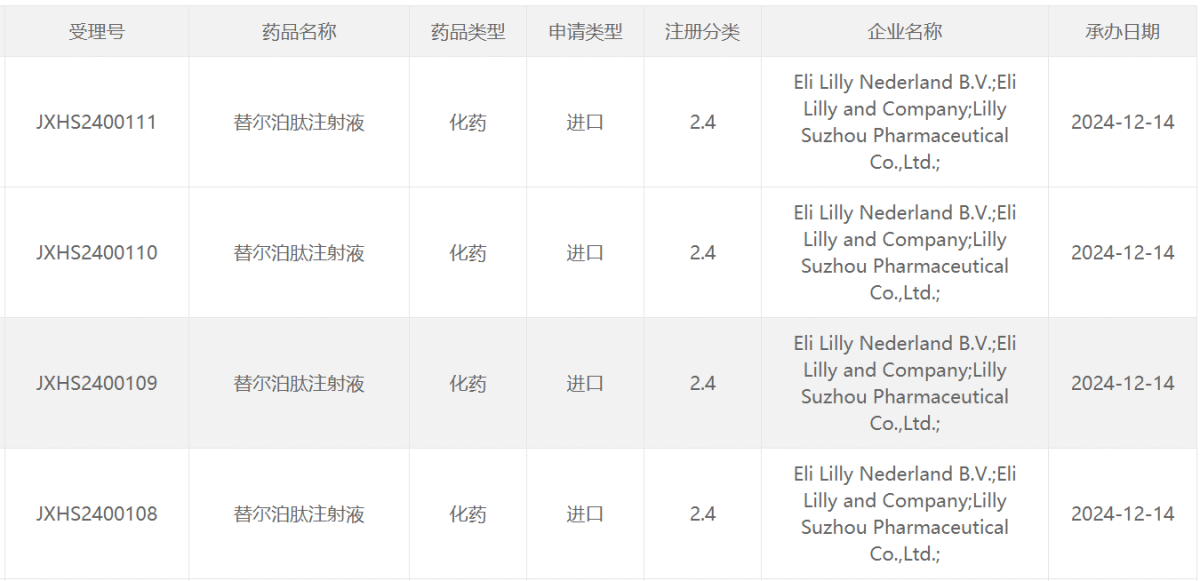
On December 14, the latest announcement on the CDE website indicated that Eli Lilly and Co (LLY.US) received acceptance for the new indication marketing application of the major drug GIP/GLP-1 receptor dual agonist tirzepatide injection.
According to Zhitong Finance APP, on December 14, the latest announcement from the website of the Drug Evaluation Center (CDE) of the National Medical Products Administration of China indicated that Eli Lilly and Co (LLY.US) received acceptance for the new indication marketing application of the major drug GIP/GLP-1 receptor dual agonist tirzepatide injection, with specific indications not yet disclosed. According to the CDE official website, this is the fifth marketing application submitted for tirzepatide in China, two of which have previously been approved by the NMPA.
Tirzepatide is a glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that is administered once a week. Currently, Eli Lilly and Co is constantly exploring the application of tirzepatide for various indications.
 According to the pipeline information on Eli Lilly and Co's official website, tirzepatide is in Phase 3 clinical trials for several indications, including reducing the incidence and mortality of obesity and decreasing adverse cardiovascular outcomes in patients with type 2 diabetes.
According to the pipeline information on Eli Lilly and Co's official website, tirzepatide is in Phase 3 clinical trials for several indications, including reducing the incidence and mortality of obesity and decreasing adverse cardiovascular outcomes in patients with type 2 diabetes.

 根据礼来官网的管线资料,替尔泊肽还针对多项适应症处于3期临床研究阶段,包括用于降低肥胖的发病率和死亡率、减少2型糖尿病患者的不良CV结局等。
根据礼来官网的管线资料,替尔泊肽还针对多项适应症处于3期临床研究阶段,包括用于降低肥胖的发病率和死亡率、减少2型糖尿病患者的不良CV结局等。