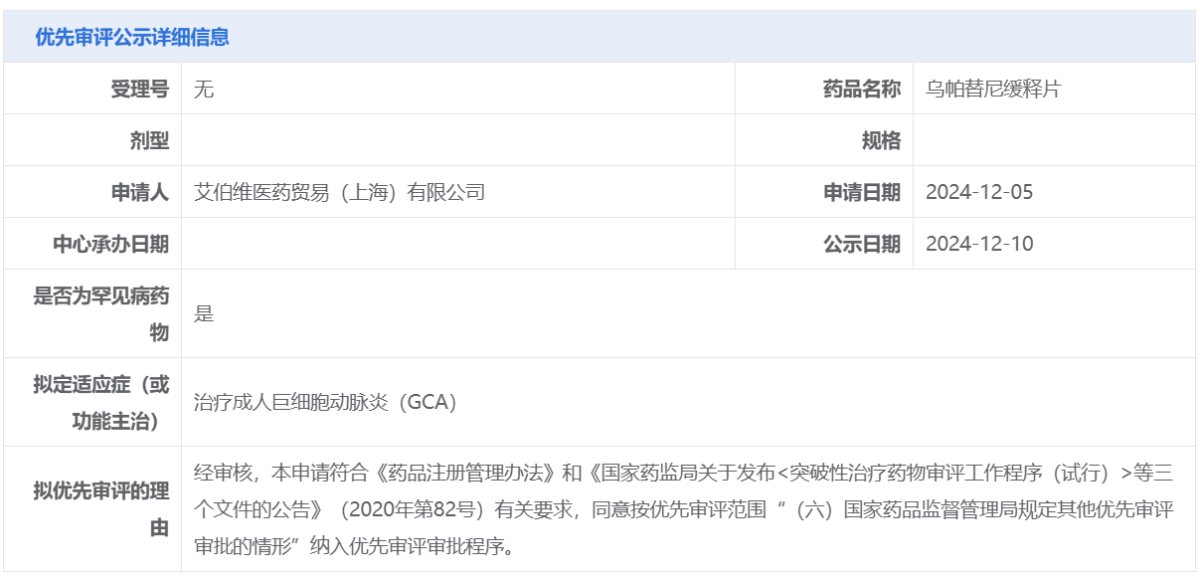
On December 10, the Center for Drug Evaluation (CDE) of China's National Medical Products Administration announced on its official website that AbbVie (ABBV.US) has submitted an application for upadacitinib sustained-release tablets to be included in priority review, with the intended indication for the treatment of adult giant cell arteritis (GCA).
According to an exclusive report from Zhito Finance APP, on December 10, the Center for Drug Evaluation (CDE) of China's National Medical Products Administration announced on its official website that AbbVie (ABBV.US) has submitted an application for upadacitinib sustained-release tablets to be included in priority review, with the intended indication for the treatment of adult giant cell arteritis (GCA). Upadacitinib (upadacitinib) is a JAK inhibitor that has previously been approved in China for the treatment of various other indications. In July of this year, AbbVie completed the submission of a new indication application for this product for the treatment of adult GCA patients to the FDA in the USA and the European Medicines Agency (EMA).
GCA is an autoimmune disease that causes inflammation of the temporal arteries and other intracranial arteries, the aorta, and other medium and large arteries. GCA usually affects elderly patients over the age of 50, with the most common age range being between 70 and 80 years. Women are at the highest risk for this disease, with symptoms that may include headaches, jaw pain, and vision changes or loss, potentially resulting in sudden and permanent vision loss.
 A previous press release from AbbVie introduced that GCA is a large vessel inflammatory disease primarily affecting the elderly, and to date, only one treatment method has been approved, which is usually used in conjunction with hormones. According to research findings, upadacitinib sustained-release tablets have the potential to become the first oral treatment option for GCA patients.
A previous press release from AbbVie introduced that GCA is a large vessel inflammatory disease primarily affecting the elderly, and to date, only one treatment method has been approved, which is usually used in conjunction with hormones. According to research findings, upadacitinib sustained-release tablets have the potential to become the first oral treatment option for GCA patients.

 艾伯维此前新闻稿介绍,GCA是一种主要影响老年人的大动脉炎症疾病,迄今为止只获批了一种治疗方法,通常与激素一起使用。根据研究结果,乌帕替尼缓释片有可能成为GCA患者的第一个口服治疗药物选择。
艾伯维此前新闻稿介绍,GCA是一种主要影响老年人的大动脉炎症疾病,迄今为止只获批了一种治疗方法,通常与激素一起使用。根据研究结果,乌帕替尼缓释片有可能成为GCA患者的第一个口服治疗药物选择。