Innovation is undoubtedly the core lifeline for the survival and development of enterprises. In today's rapidly changing business environment, companies must continuously innovate and achieve self-breakthroughs to maintain competitiveness.
As Darwin said, "It is not the strongest species that survive, nor the most intelligent, but the one most responsive to change." Therefore, how companies strengthen their core competitiveness and continuously enhance their technology capabilities around innovation to lead industry development and even drive industrial revolution will become the key to their emergence.
Recently, at the "Gelonghui·Global Investment Carnival·2025" event, Xinwei Medical was awarded the "Annual Innovation Award," showcasing its outstanding innovative strength in multiple dimensions such as product innovation, model innovation, and organizational innovation, which also reflects the market's high recognition of its innovative capabilities.

 On the day of the event, Xinwei Medical engaged in in-depth communication and sharing with investors. This open communication method not only helps investors gain a deeper understanding of the listed company but also provides them with valuable opportunities to capture future investment opportunities in the innovative medical instruments industry. The author has also organized the speech content from Xinwei Medical, and from a personal perspective, Xinwei Medical's innovative practices offer some experiences worth learning for other enterprises, which investors may find insightful.
On the day of the event, Xinwei Medical engaged in in-depth communication and sharing with investors. This open communication method not only helps investors gain a deeper understanding of the listed company but also provides them with valuable opportunities to capture future investment opportunities in the innovative medical instruments industry. The author has also organized the speech content from Xinwei Medical, and from a personal perspective, Xinwei Medical's innovative practices offer some experiences worth learning for other enterprises, which investors may find insightful.
Comprehensive layout of neurointervention, interpreting differentiated innovation.
As a leading domestic neurointerventional medical instruments enterprise, Xinwei Medical always focuses on meeting the needs gap of clinical doctors and patients. By continuously launching innovative medical instruments, it redefines care standards, reduces mortality rates, and improves prognosis, providing the first domestic one-stop solution for stroke treatment and prevention.
So, how does Xinwei Medical interpret innovation? We can analyze it from three simple dimensions.
Firstly, from the perspective of business layout, Xinwei Medical demonstrates its comprehensive layout and innovative capabilities in the field of neuro-interventional medical instruments.
Currently, the company has 30 medical device products approved by the NMPA, three medical device products approved by the FDA, and one product that has obtained the CE mark, broadly covering areas such as acute ischemic stroke and neurovascular stenosis treatment, hemorrhagic stroke treatment, ischemic stroke prevention, interventional pathways, and peripheral interventional devices, fully reflecting its deep understanding of the market demand for neuro-interventional medical instruments.
At the same time, the company's expansion in overseas markets has also yielded remarkable results, with its thrombectomy brackets, occlusion balloon catheters, remote access catheters, and micro catheters all achieving CE or FDA certification, and registrations completed in countries or regions such as Thailand, officially initiating the commercialization process.
Of course, to achieve its long-term overseas sales goals, Xinwei Medical is actively carrying out product registration work in more than 10 other countries or regions, continuously expanding its sales pipeline. This is not only an important step in the company's international strategy but also provides strong support for its competitiveness in the global market.
Secondly, from the development strategy of its core business, Xinwei Medical has shown strategic vision in diligently cultivating and pursuing differentiated competition in the field of neuro-intervention.
Since the end of last year, the company has noticed the increasingly severe homogenization of access products. To better adapt to the rapidly changing market environment, Xinwei Medical continuously promotes the transformation and upgrade of its neuro-interventional business towards differentiated therapeutic devices, focusing its R&D efforts on user solutions centered around therapeutic products. This strategic adjustment not only provides strong support for the growth of company product sales but also highlights its innovative capabilities and market adaptability in the field of neuro-intervention.
For example, in response to the emergency surgical needs for different subtypes of cerebral infarction, the company focuses on market orientation, enhancing the competitiveness of key thrombectomy products (suction catheters and thrombectomy brackets) and one-stop medical device solutions to meet the growing demand for stroke treatment in the Chinese market against the backdrop of an aging population.
Finally, the innovation of the products is noteworthy.
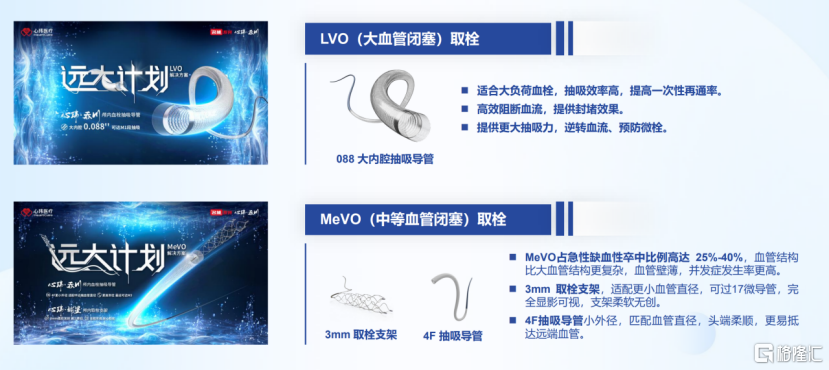
In the latest product layout, Xinwei Medical has proposed a "Grand Plan" for thrombectomy treatment medical instruments, including two innovative products: LVO (large vessel occlusion) thrombectomy and MeVO (medium vessel occlusion) thrombectomy.
The LVO thrombectomy is specifically designed for handling large-load thrombosis, featuring higher suction efficiency, which can significantly enhance the first-time recanalization rate. This product blocks blood flow efficiently to provide occlusion effects and reverses blood flow by enhancing suction power, thereby effectively preventing the formation of microemboli.
The MeVO thrombectomy uses an innovative suction catheter combined with bracket withdrawal technology, which allows for higher transport rates and prognosis rates. Compared to singular bracket thrombectomy or suction thrombectomy methods, it demonstrates superior therapeutic effects.
Previously, at the mid-term earnings conference, Xinwei Medical gave a positive forecast regarding the commercialization process of subsequent products, expecting that within the next 18 months, at least five major neurointerventional medical devices will be launched, including intracranial drug-eluting balloon catheters for stenosis treatment, self-expanding drug brackets, carotid brackets for hemorrhagic stroke treatment, along with aneurysm embolization assisting brackets and flow-directed devices.
According to the current process, the aneurysm embolization assisting bracket has been approved for market launch, making it the first domestically produced and currently the only aneurysm embolization assisting bracket. Everything is progressing as planned. This orderly commercialization process also reflects Xinwei Medical's efficient execution in product development and market promotion, providing strong support for its future market performance.
Therefore, the comprehensive commercial layout, differentiated core business development strategy, and continuous innovation in product development fully demonstrate Xinwei Medical's innovative strength and market competitiveness in the neurointerventional medical device field, continuously providing the company with sustainable growth momentum.
Financial indicators have improved significantly, verifying innovative results.
The comprehensive improvement in financial levels further verifies the innovative results and value of Xinwei Medical, undoubtedly providing the company with more development opportunities and advantages in the fierce market competition.
According to data, the company achieved revenue of 0.128 billion yuan in the first half of the year, a year-on-year increase of 17.24%. This growth is mainly attributed to the strong performance of devices for acute ischemic stroke (AIS) thrombectomy, intracranial artery stenosis treatment devices, and innovative channel devices.
In addition, thanks to the approval of multiple product registrations by local drug regulatory authorities, the company's overseas revenue has also been effectively boosted, indicating that Xinwei Medical's layout in the international market is gradually showing results, and the international competitiveness of its products has been further verified.
On one hand, the number of implanted neurointerventional therapy devices, such as thrombectomy brackets, aspiration catheters, balloon dilators, embolization protection systems, and coils in hospitals continues to rise, showcasing the popularity and high recognition of its innovative products in the market. At the same time, the sales of neurointerventional channel devices and other products increased by 42.1% year-on-year, further consolidating the company’s leading position in the industry. This diversified source of income not only enhances the company's market stability but also provides a solid foundation for its future growth.
In terms of profitability, the company's pre-tax losses in the first half of the year further narrowed to 3.2 million yuan, a year-on-year decrease of 94.1%. The improvement in profitability is a direct reflection of the company's innovative results, with an expectation to achieve breakeven throughout the year.

Conclusion
From the current perspective, as an industry leader, the growth potential behind Xinwei Medical's innovation should not be underestimated.
In fact, from an industry perspective, besides building core capabilities, Xinwei Medical's foresight and adaptability in the industry's development trends are also key to its continued prominence.
In the short term, favorable policies have recently begun to emerge intensively in the field of medical instruments, supporting the recovery and improvement of the company's performance.
For example, in September, Li Li, the director of the National Medical Products Administration, proposed to deepen drug supervision reform, aiming to speed up the market access of innovative drugs and medical devices and improve the quality of pharmaceutical industry development; in October, the notice on the application for the promotion and application of high-end medical equipment projects in 2024 emphasized the promotion of high-end medical equipment, covering diagnostics, treatment, monitoring, rehabilitation, and ai-assisted diagnosis and treatment, among other fields. In November, the National Development and Reform Commission emphasized support for industrial transformation and upgrading, with equipment update policies focusing on seven key areas including medical to facilitate the transition of domestic substitution from mid- to low-end to high-end and solve the "bottleneck" problem.
In the long term, the increasing aging population in our country will drive steady growth in medical demand, indicating a broad long-term development space for the industry, which also means that enterprises like Xinwei Medical have a high growth ceiling.
According to Frost & Sullivan data, it is estimated that by 2025, the market size of neurointerventional devices in china will reach 15.842 billion yuan, with a compound annual growth rate of 23.13% from 2021 to 2025, and it is expected to rise to about 44.226 billion yuan by 2030.
Overall, innovation is an inexhaustible driving force for the sustainable development of enterprises. The case of Xinwei Medical again proves that whether it is the continuously innovating and upgrading products, greatly improved financial performance, or the steadily growing market demand driven by aging trends, these positive factors constitute a solid foundation for the company’s rapid development in high-end innovative medical instruments, while also providing ample support for winning the "Annual Innovation Award" label recognized by investors and the market.
With the winning of this award, it is believed that the market's attention and expectations for Xinwei Medical will also be further enhanced.
Below is the Q&A section of this roadshow:
Q1: What is the difference in sales of the company's products between china and overseas? What are the avenues for developing overseas markets?
A1: The sales of Xinwei Medical's products abroad are mainly conducted through dealers rather than self-developing the market. Currently, most domestic medical instrument companies rely on dealers to explore overseas markets. Almost all products in the company's pipeline are exported, among which the distal access catheter is the product with the highest sales; it is also the main general medical device for thrombectomy. Other products like brackets and suction catheters are also gradually increasing in sales.
Q2: Some investors mentioned a slight decrease in research and management costs last year. Will this affect the future pipeline development?
A2: Xinwei Medical has laid out numerous product lines in the early stages, and 2021 was the first year of commercialization for the group. After continuous internal sorting and adjustment in 2023 and 2024, the company is currently focused on the neurointerventional field, especially in thrombectomy and stenosis, aiming to become the market leader among domestic brands. In this process, the company will gradually eliminate pipelines that are less related to the main business or have low short-term return on investment, and later compensate or expand the product portfolio through incubation or external acquisitions. The reduction in management costs is mainly achieved through measures that promote cost reduction and efficiency enhancement within the company.
Q3: How to view the impact of centralized procurement policies on the company, and what are the company's expectations for future centralized procurement policies?
A3: Centralized procurement policies are an unavoidable topic in the entire pharmaceutical industry. For enterprises, although the decrease in product prices will affect revenue to some extent, outstanding domestic medical devices can quickly penetrate markets previously dominated by foreign brands through volume procurement organized by national or inter-provincial alliances, providing high-quality products for patients while reducing economic burdens and improving product accessibility.
Many products in the neurointerventional field have already been included in centralized procurement, and only a few products have not yet entered large-scale procurement. The company expects 2025 to be a significant year for neurointerventional centralized procurement, with products entering centralized procurement next year. The company will prepare adequately before centralized procurement and actively respond in hopes of achieving good results during bidding. Through centralized procurement, the company aims to achieve import substitution and quickly enter the market. Taking the carotid artery stenosis market as an example, foreign products are expected to have a market share exceeding 90%, hence there is immense market potential for outstanding domestic brands to achieve import substitution.

 活动当天,心玮医疗与投资者进行了深入的交流与分享。这种开放的沟通方式不仅有助于投资者更深入地了解上市公司,也为他们提供了捕捉未来创新医疗器械行业投资机会的宝贵机会。笔者也整理了这次心玮医疗的演讲内容,从个人角度来看,心玮医疗的创新实践也为其他企业提供了一些值得借鉴的经验,或许投资者能在其中找到答案。
活动当天,心玮医疗与投资者进行了深入的交流与分享。这种开放的沟通方式不仅有助于投资者更深入地了解上市公司,也为他们提供了捕捉未来创新医疗器械行业投资机会的宝贵机会。笔者也整理了这次心玮医疗的演讲内容,从个人角度来看,心玮医疗的创新实践也为其他企业提供了一些值得借鉴的经验,或许投资者能在其中找到答案。