Astrazeneca (AZN.US) applied for a Class 1 new drug AZD5462 tablet in China and obtained an implied clinical trial approval to develop the treatment for heart failure.
According to the Financial Intelligence APP, today (November 29th), the latest announcement on the official website of the National Medical Products Administration's Center for Drug Evaluation (CDE) in China stated that Astrazeneca (AZN.US) has obtained an implied approval for clinical trials of the Class 1 new drug AZD5462 tablet, aiming to develop the treatment for heart failure. According to information on the Astrazeneca website, this is an RXFP1 agonist small molecule drug, currently in phase 2 clinical research internationally. As per the CDE official website search, this is the first time this drug has been approved for clinical use in China.
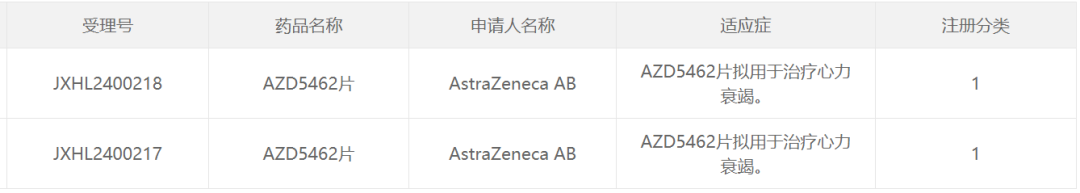
Screenshot source: CDE official website
 It is reported that RXFP1 is a relaxin receptor, which is widely expressed in many organs and tissues including the heart, kidneys, and blood vessels. Relaxin is an endogenous insulin-like peptide with beneficial physiological effects in the cardiovascular system and good safety.
It is reported that RXFP1 is a relaxin receptor, which is widely expressed in many organs and tissues including the heart, kidneys, and blood vessels. Relaxin is an endogenous insulin-like peptide with beneficial physiological effects in the cardiovascular system and good safety.
According to the ClinicalTrials official website, Astrazeneca is conducting a phase 2b clinical study of AZD5462, evaluating the product's impact on the heart function of chronic heart failure (HF) patients. The study plans to enroll 360 participants globally and is scheduled to be completed in November 2025. The approval of AZD5462 for clinical use in China means that it will enter the clinical development phase in China.

 据悉,RXFP1是一种松弛素受体,该受体在许多器官和组织中广泛表达,包括心脏、肾脏和血管等。松弛素(Relaxin)是一种内源性异二聚胰岛素样肽,在心血管系统中具有有益的生理作用,并且具有较好的安全性。
据悉,RXFP1是一种松弛素受体,该受体在许多器官和组织中广泛表达,包括心脏、肾脏和血管等。松弛素(Relaxin)是一种内源性异二聚胰岛素样肽,在心血管系统中具有有益的生理作用,并且具有较好的安全性。