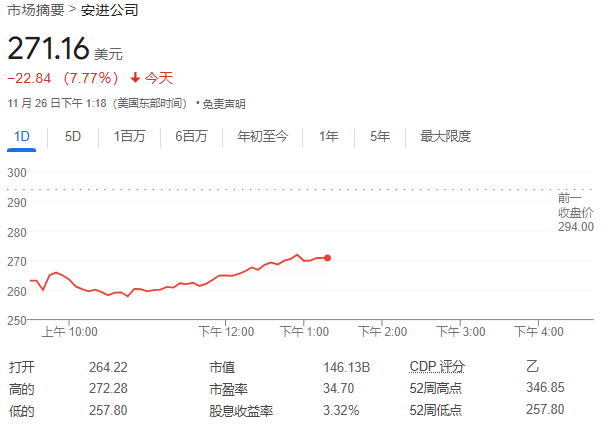
Although the weight loss effect of amgen's MariTide drug met expectations, the high side effects, lower than eli lilly and co's Zepbound, and the trial results falling below Wall Street's high expectations, led to amgen's stock price plunging over 12.3% on Tuesday, far behind eli lilly and co and novo-nordisk a/s, which benefited from the Biden policy rise at the same time. In a one-year trial, MariTide helped non-diabetic patients lose up to 20% of their weight, comparable to the maximum dose effect of eli lilly's Zepbound. However, the side effects are significant, with about 11% of patients dropping out of the trial due to side effects, higher than eli lilly's 7%.
On Tuesday, as the Biden administration plans to include weight loss drugs in Medicare and subsidy programs, $Novo-Nordisk A/S (NVO.US)$ rose more than 3% at one point, $Eli Lilly and Co (LLY.US)$ rose nearly 6.9% at one point.
Amidst the widespread rise of weight loss drug companies, $Amgen (AMGN.US)$ Once fell by more than 12.3%, the reason was that although the efficacy of Amgen's MariTide weight loss drug met Wall Street's expectations, it failed to beat Eli Lilly's blockbuster drug Zepbound due to higher side effects, and the trial results fell below Wall Street's high expectations for MariTide. Before the data was released, some analysts even hoped for a weight loss of 25%.
 Amgen stated that in a year-long trial, non-diabetic patients taking Amgen's MariTide once a month or less frequently lost a maximum of 20% of their weight. Late-stage studies with Wegovy showed a 15% weight loss within 68 weeks, while Zepbound helped patients lose over 22% of their weight in 72 weeks.
Amgen stated that in a year-long trial, non-diabetic patients taking Amgen's MariTide once a month or less frequently lost a maximum of 20% of their weight. Late-stage studies with Wegovy showed a 15% weight loss within 68 weeks, while Zepbound helped patients lose over 22% of their weight in 72 weeks.
Analysts point out that this is comparable to the maximum dose effect of Eli Lilly's Zepbound. However, both Zepbound and Novo Nordisk's Wegovy are injected once a week, so MariTide may have an advantage over the popular weekly injection treatments on the market.
However, data shows that MariTide has more side effects compared to Zepbound. Around 11% of patients dropped out of the MariTide trial due to side effects, while in the phase 3 trials of Zepbound, this proportion was around 7%. These side effects are mainly gastrointestinal in nature, as expected for this type of drug.
Amgen responded that nausea and vomiting are usually mild and short-lived, mainly related to the initial use of the drug. The company addressed these symptoms by slowing down the administration time.
Amgen's advantages: lower treatment frequency, brand-new mechanism of action.
It is worth noting that Amgen further stated that patients taking the highest dose of MariTide once every two months have similar weight loss effects to those taking it once a month, indicating the drug may have the potential for lower dosing frequency.
Furthermore, MariTide uses a new approach compared to existing drugs on the market. The weight loss drug MariTide from Amgen employs a mechanism different from Eli Lilly and Co's Zepbound. Zepbound enhances satiety and lowers blood sugar indicators by simulating two hormones (GLP-1 and GIPR), while MariTide simulates GLP-1 and inhibits GIPR activity.
Moreover, MariTide users did not experience a "plateau phase" during the weight loss process. Analyst Gregory Renza from RBC Capital Markets mentioned in a report,
"This may indicate that the combination mechanism of GLP-1 agonist and GIPR antagonist provides more convenient administration and may lead to more long-term weight maintenance effects, which is an important difference of MariTide compared to other GLP-1 drugs."
It is noteworthy that Amgen also stated that weight loss in type 2 diabetes patients can reach up to 17% without a plateau phase, indicating the potential for further weight loss after 52 weeks. At the same time, their glycated hemoglobin (HbA1c) levels, an indicator measuring changes in blood sugar levels over time, decreased by 2.2%.
Additionally, patients showed improvements in cardiac metabolic indicators, including blood pressure, triglycerides, and high-sensitivity C-reactive protein (hs-CRP).
Amgen also pointed out that after using MariTide, patients did not show a significant increase in free fatty acids levels. In contrast, analyst David Risinger from Leerink Partners mentioned that in a pooled analysis of two studies on Eli Lilly and Co's Zepbound, free fatty acid levels decreased by about 7.5%. Free fatty acids are released by adipose tissue (fat cells) and are closely associated with insulin resistance and heart disease.
It is worth mentioning that Amgen also stated that no evidence of bone loss was found among MariTide users. Previously, on November 12, there were reports that early tests suggested a possible link between MariTide and bone loss, which caused a sharp drop in Amgen's stock price.
Amgen's CEO, Robert Bradway, stated during an investor conference call on Tuesday:
"Based on this data, we believe MariTide has unique differentiation and competitive advantages, and we will further explore these advantages in Phase III development."
Furthermore, Amgen also announced the initiation of the second part of the trial, which will explore lower doses and less frequent dosing regimens. Renza noted that approximately 90% of patients are continuing to participate in this part of the trial. Amgen also announced the initiation of its Phase III trial named Maritime.
Amgen stated that MariTide may offer faster weight loss effects than novo-nordisk a/s's Wegovy and Eli Lilly and Co's Zepbound, which are injected once a week, potentially better weight maintenance, and fewer injection frequencies. Analysts pointed out that this may increase Amgen's chance to grab a share in the weight loss drug market. According to some analysts' predictions, the annual value of the weight loss drug market could reach as high as $150 billion by the early 2030s.
Editor / jayden

 安进表示,在为期一年的试验中,非糖尿病患者每月或更少频次服用一次安进的MariTide,最多减轻了20%的体重。Wegovy的后期研究显示,在68周内实现了15%的体重减轻,而Zepbound在72周内帮助患者减轻了超过22%的体重。
安进表示,在为期一年的试验中,非糖尿病患者每月或更少频次服用一次安进的MariTide,最多减轻了20%的体重。Wegovy的后期研究显示,在68周内实现了15%的体重减轻,而Zepbound在72周内帮助患者减轻了超过22%的体重。