Nuclear medicine, represented by radioligand therapy, is an area heavily developed by Novartis.
The Zhitong Finance App learned that on November 13, Novartis (NVS.US) lutetium [177Lu] terciviropitide marketing application was accepted by the CDE. Previously, the drug was included in the CDE priority review and is suitable for the treatment of prostate-specific membrane antigen (PSMA) -positive metastatic castration-resistant prostate cancer (mCRPC), adult patients who have received androgen receptor pathway suppression and yew chemotherapy. This drug is the first targeted radioligand therapy approved by the FDA for patients with castration-resistant prostate cancer. This is the first time it has been reported and marketed in China.
Nuclear medicine, represented by radioligand therapy, is an area heavily invested by Novartis. In addition to Pluvicto, Novartis also has a nuclear drug, Lutathera, which has also been approved for marketing in adult patients with somatostatin receptor-positive (SSTR+) gastrointestinal and pancreatic neuroendocrine tumors, and later approved for use in children 12 years and older. Lutathera had sales of $0.605 billion in 2023 and $0.534 billion in the first three quarters of 2024.
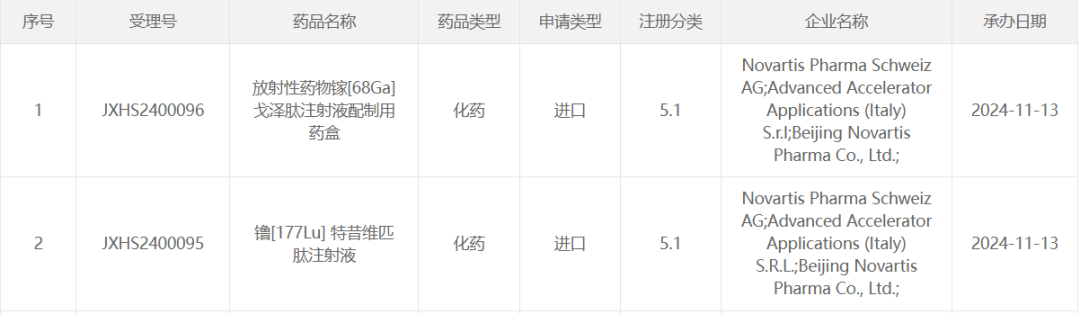
 Screenshot source: CDE official website
Screenshot source: CDE official website

 截图来源:CDE 官网
截图来源:CDE 官网