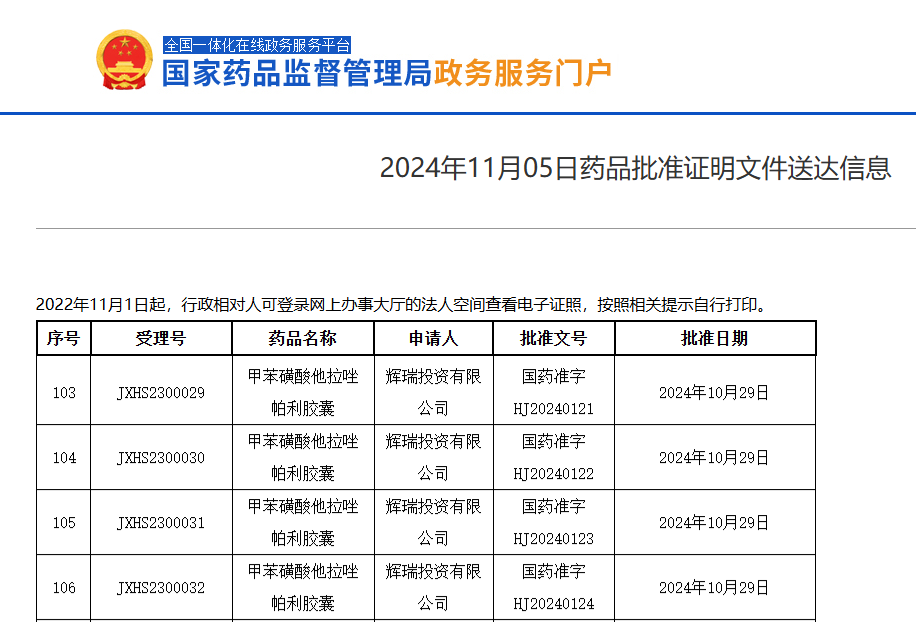
On November 5, the official website of the National Medical Products Administration (NMPA) showed that Pfizer's talazoparib tosylate capsules (talazoparib) have been approved for marketing.
According to the Securities Times APP, on November 5, the official website of the National Medical Products Administration (NMPA) showed that Pfizer's talazoparib tosylate capsules (talazoparib) have been approved for marketing. Talazoparib was originally developed by BioMarin, acquired by Medivation from BioMarin in 2015, and Pfizer acquired Medivation for $14 billion in 2016, thus talazoparib belongs to Pfizer since then.
Talazoparib is a poly adenosine diphosphate ribose polymerase (PARP) inhibitor, including PARP1 and PARP2. In vitro studies indicate that for tumor cell lines with DNA repair gene defects (including BRCA1 and BRCA2), based on the Synthetic Lethality principle, talazoparib may exert cytotoxic effects by inhibiting PARP enzyme activity and promoting the formation of PARP-DNA complexes, leading to tumor cell DNA damage, inhibition of tumor cell proliferation, and promotion of tumor cell apoptosis.
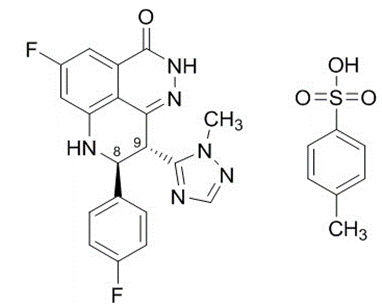
 In 2018, the FDA approved talazoparib for the treatment of HER2-negative locally advanced or metastatic breast cancer with germline BRCA1/2 mutations (gBRCAm), and later also approved for the treatment of metastatic castration-resistant prostate cancer (mCRPC). Previously, domestically, four PARP inhibitors, namely Olaparib (AstraZeneca), Fluzoparib (Hengrui), Niraparib (GSK/Chengdu Dingtai), Pamiparib (BeiGene), have been approved for marketing. In addition, Johnson & Johnson's Niraparib Acetate Abiraterone compound has also been approved for marketing. Another PARP inhibitor, Senaparib (Senaparib, Innovent/Huadong Medicine), has been applied for marketing and is currently under review.
In 2018, the FDA approved talazoparib for the treatment of HER2-negative locally advanced or metastatic breast cancer with germline BRCA1/2 mutations (gBRCAm), and later also approved for the treatment of metastatic castration-resistant prostate cancer (mCRPC). Previously, domestically, four PARP inhibitors, namely Olaparib (AstraZeneca), Fluzoparib (Hengrui), Niraparib (GSK/Chengdu Dingtai), Pamiparib (BeiGene), have been approved for marketing. In addition, Johnson & Johnson's Niraparib Acetate Abiraterone compound has also been approved for marketing. Another PARP inhibitor, Senaparib (Senaparib, Innovent/Huadong Medicine), has been applied for marketing and is currently under review.

 2018年,FDA批准他拉唑帕利治疗胚系突变BRCA1/2(gBRCAm)的HER2阴性局部晚期或转移性乳腺癌,后也被批准用于治疗转移性去势抵抗性前列腺癌(mCRPC)。此前,国内已有奥拉帕利(阿斯利康)、氟唑帕利(恒瑞)、尼拉帕利(GSK/再鼎)、帕米帕利(百济)这4款PARP抑制剂获批上市,以及强生的尼拉帕利醋酸阿比特龙复方也已获批上市。另外还有一款PARP抑制剂塞纳帕利(senaparib,英派药业/华东医药)已申报上市,正在审评当中。
2018年,FDA批准他拉唑帕利治疗胚系突变BRCA1/2(gBRCAm)的HER2阴性局部晚期或转移性乳腺癌,后也被批准用于治疗转移性去势抵抗性前列腺癌(mCRPC)。此前,国内已有奥拉帕利(阿斯利康)、氟唑帕利(恒瑞)、尼拉帕利(GSK/再鼎)、帕米帕利(百济)这4款PARP抑制剂获批上市,以及强生的尼拉帕利醋酸阿比特龙复方也已获批上市。另外还有一款PARP抑制剂塞纳帕利(senaparib,英派药业/华东医药)已申报上市,正在审评当中。