①Novo Nordisk announced the Phase III trial Phase I results of semaglutide 2.4 mg for significant improvement in liver fibrosis and MASH; ②The trial showed that 37.0% of patients in the semaglutide group had improved liver fibrosis and no worsening of fatty liver hepatitis, compared to 22.5% in the placebo group; ③Novo Nordisk expects to submit regulatory approval to the USA and the EU in the first half of 2025, with MASH affecting approximately 5% of adults in the USA.
On November 2, Cailian News Agency (edited by Zhao Hao) reported that local time on Friday, November 1, Novo Nordisk announced on its official website the Phase I results of an ongoing Phase III trial, showing significant improvement in liver fibrosis and MASH regression with semaglutide 2.4 mg.

Nonalcoholic steatohepatitis (NASH), formerly known as metabolic dysfunction associated fatty liver disease (MASLD), progresses from metabolic dysfunction-associated fatty liver disease. Patients with NASH often have fatty degeneration, inflammation, and liver fibrosis, which can further develop into cirrhosis.
Without timely treatment, the risk of cirrhosis increases, leading to liver failure and liver cancer, ultimately resulting in death. Therefore, the degree of fibrosis improvement in patients in clinical trials of MASH drugs is a commonly used primary endpoint.
Due to its complex pathogenesis, it has been a research and development black hole in the pharmaceutical industry for a long time, with many pharmaceutical companies experiencing repeated failures.
Novo Nordisk's 'ESSENCE' is a critical Phase III, 240-week double-blind trial that includes 1,200 adults with MASH and moderate to advanced liver fibrosis. They were randomized in a 2:1 ratio to receive 2.4mg of semaglutide or placebo on top of standard treatment.
The trial achieved its primary endpoint: at week 72, 37.0% of patients in the semaglutide group had improved liver fibrosis and no worsening of fatty liver hepatitis, compared to 22.5% in the placebo group; 62.9% of patients in the semaglutide group had alleviated fatty liver disease and no worsening of liver fibrosis, compared to 34.1% in the placebo group.
Semaglutide also demonstrated safety and good tolerability, as stated by Martin Holst Lange, Executive Vice President of Research and Development at Novo Nordisk, 'We are very pleased with the results of the ESSENCE clinical trial and the potential of semaglutide to help MASH patients.'
Novo Nordisk is expected to submit regulatory approvals to the USA and the EU in the first half of 2025. Lange added, "One third of overweight or obese individuals have MASH. This has a serious impact on their health, representing an unmet significant need."
Following the data release, there was little change in Novo Nordisk's stock price in the U.S. market; whereas Madrigal Pharmaceuticals surged over 19%, reaching nearly 24% at its peak, due to its drug Rezdiffra being the first and only MASH drug in the USA.
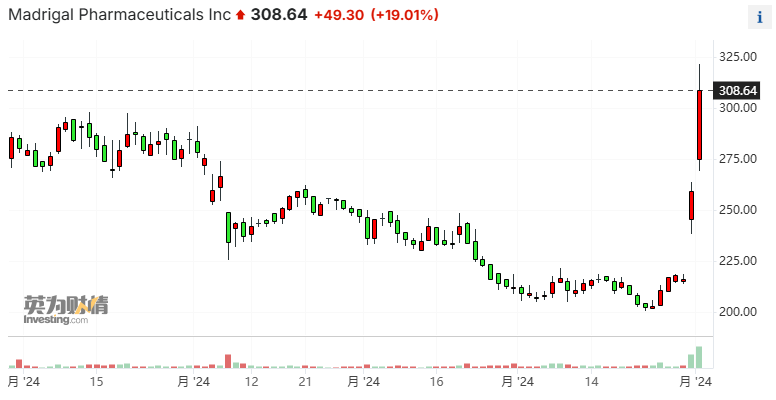
Rezdiffra is the first MASH drug approved by the FDA for market launch in 40 years. Piper Sandler analyst Yasmeen Rahimi is bullish on Madrigal's long-term prospects, "because large pharmaceutical companies have the resources to significantly expand this market and create greater opportunities for Rezdiffra."
According to data from the American Liver Foundation, MASH affects approximately 5% of adults in the USA, making the patient group very large. In June of this year, Eli Lilly also announced positive results from the phase two clinical trial of tirzepatide for treating MASH.

 如果不及时治疗,肝硬化风险将增加,诱导患者出现肝衰竭和肝癌,最终导致死亡。因此在MASH药物的临床试验中患者纤维化改善程度是常用的主要终点。
如果不及时治疗,肝硬化风险将增加,诱导患者出现肝衰竭和肝癌,最终导致死亡。因此在MASH药物的临床试验中患者纤维化改善程度是常用的主要终点。