The Federal Reserve announced a 50 basis point rate cut on September 18, 2024, marking a shift in the Fed's monetary policy from a tightening cycle to an easing cycle. At the same time, the Chinese government also introduced a series of fiscal and monetary policies to boost domestic demand, including issuing ultra-long special national bonds and measures such as reserve requirement ratio cuts and interest rate cuts by the central bank. The implementation of these policies released liquidity, providing support for the capital markets.
Against this background, the healthcare sector in the Hong Kong stock market was buoyant and performed well in this round of rebound. In the third quarter of 2024, the fund holding ratio of the pharmaceutical industry continued to rise, with pharmaceutical funds reaching 314.1 billion yuan, a 15% increase month-on-month. In addition, the Hang Seng Healthcare ETF also showed a clear upward trend. This further confirms the market's positive attitude towards the healthcare sector of Hong Kong stocks.
It is worth mentioning that the on-site negotiations of the medical insurance directory started on October 27, with the final results expected to be announced in November. Innovative drugs and traditional Chinese medicine are the key supported directions in this round of medical insurance directory, and related sectors are expected to see another boost.
 Against this backdrop, Hong Kong stocks welcomed Hua Hao Zhong Tian Medicine-B (2563.HK), the 'first stock of synthetic biology medicine.' According to the announcement, the company plans to globally issue 14.588 million shares, issue 1.4588 million shares in Hong Kong, and issue 13.1292 million shares internationally, with a final offering price of HK$16 per share.
Against this backdrop, Hong Kong stocks welcomed Hua Hao Zhong Tian Medicine-B (2563.HK), the 'first stock of synthetic biology medicine.' According to the announcement, the company plans to globally issue 14.588 million shares, issue 1.4588 million shares in Hong Kong, and issue 13.1292 million shares internationally, with a final offering price of HK$16 per share.
This article will explore the investment value of Hua Hao Zhong Tian from the perspectives of imaginative space, competitiveness, and growth prospects.
Race full of imaginative space
Hua Hao Zhong Tian focuses on the development of innovative cancer drugs based on synthetic biology technology. The company relies on an advanced synthetic biology new drug research platform to develop proprietary anti-cancer new drugs. Its core product, Autidolon, is the world's first chemotherapeutic new molecule developed and listed based on synthetic biology technology.
With the advancement of population aging and urbanization in China, the number of cancer patients continues to increase, and the demand for anti-tumor drugs in the market is constantly rising. It is expected by The Economist Intelligence Unit that by 2029, the market size of the anti-tumor drug industry in China is expected to reach 559 billion yuan, with a compound annual growth rate of approximately 19% from 2024 to 2029.
Chemotherapy drugs play a crucial role in clinical treatment due to their broad-spectrum anti-tumor activity. They not only demonstrate significant efficacy in various types of cancer, but they can also be used in different stages of cancer treatment, including early neoadjuvant therapy, adjuvant therapy, and late-line therapy. Chemotherapy drugs have become an indispensable foundational treatment in cancer treatment plans. In addition, with the increase in tumor resistance, the effectiveness of targeted therapy may decrease, leading many cancer patients to ultimately rely on chemotherapy drugs. As the field of cancer treatment advances and new drugs emerge, there is a growing trend in clinics towards adopting treatment strategies that combine chemotherapy drugs to enhance treatment efficacy.
According to CCID Consulting's statistics, it is expected that by 2030, the market size of chemotherapy drugs in China is expected to reach 170.7 billion yuan. Roughly estimated, this accounts for one-third of the overall market size of anti-tumor drugs. Paclitaxel is one of the most widely used chemotherapy drugs. However, in the past decade, globally, there have been no breakthrough chemotherapy drugs launched besides paclitaxel, and traditional chemotherapy drugs are prone to resistance. Hua Hao Zhong Tian's Yutaidaolong injection is the first class of innovative chemotherapy drugs independently developed in China in nearly twenty years. It is also the only approved microtubule inhibitor with a new molecular structure globally in over a decade.
First-class innovative chemotherapy drugs have brand-new chemical structures and advantages in efficacy and safety, which means they can provide patients with completely new treatment options. The successful market launch of Yutaidaolong injection has broken through the long-term bottleneck in the treatment of advanced breast cancer in China.
An innovative drug project with a large market capacity and unmet clinical needs typically has high value.
Looking at it from a more microscopic perspective, Yutaidaolong has a similar mechanism of action to taxane drugs, both being microtubule stabilizers. However, Yutaidaolong's unique binding site with microtubule proteins and its dual targeting mechanism give it characteristics such as stronger activity, broader spectrum of anti-cancer effects, lower hematotoxicity, and reduced likelihood of developing resistance. Currently, apart from the indications for recurrent or metastatic advanced breast cancer that have been approved for market launch, Yutaidaolong injection is conducting several domestic and international clinical studies in indications such as neoadjuvant breast cancer, late-stage non-small cell lung cancer (NSCLC), advanced stomach and esophageal cancer, ovarian cancer, bile duct cancer, breast cancer brain metastases, lung cancer brain metastases, among others.
This belongs to the field of broad indications and rare varieties, with ample imagination for pipeline value.
Building a broad competitive moat.
When evaluating the core competitiveness of innovative drug companies, the technological platform is a crucial consideration factor. The technology platform not only constitutes the foundation of innovative drug research and development, but also directly affects the speed, cost, drug quality, and efficacy of research and development.
Huahao Zhongtian has successfully developed three core technological platforms focusing on the research and development of microbial metabolite new drugs, including the combination biosynthesis platform, microbial fermentation production technology platform, and microbial drug formulation development platform.
First, the combination biosynthesis platform allows Huahao Zhongtian to apply synthetic biology technology to the molecular discovery stage.
Synthetic biology can promote the rapid and accurate synthesis of DNA fragments, genes, or gene libraries, explore and discover better clinical utility targets, and ultimately produce innovative drug molecules that may have better safety and efficacy. The molecular discovery stage is crucial for innovative drug research and development, being the primary stage. This stage competes on the efficiency of the technological platform, i.e., how to quickly and cost-effectively screen out molecules with the strongest target effects. Huahao Zhongtian's combination biosynthesis platform, through targeted modification of biosynthetic gene clusters or altering microbial metabolic pathways, enables the company to better design and manufacture "unnatural natural compounds," improving the efficiency of the molecular discovery stage.
Secondly, the microbial fermentation production technology platform. This platform not only possesses environmentally friendly characteristics but also provides Huahao Zhongtian with the assurance of stable green production. On this platform, the company has successfully industrialized drug production through microbial fermentation, establishing a complete system for industrial production of microbial metabolite innovative drugs, providing reliable support for the continuous development of innovative drugs.
Lastly, the microbial drug formulation development platform, which focuses on the research and optimization of microbial drug formulations. Through this platform, Huahao Zhongtian can develop drug formulations more suitable for clinical application, thereby improving drug efficacy and patient compliance. The company can utilize differentiated formula designs, preparation methods, production processes, and critical quality attribute controls to develop diversified drug dosage forms, thereby improving the drugability of microbial small molecule compounds, enhancing the convenience, safety, and effectiveness of clinical drug application.
The three efficient and advanced technological platforms empower each other, accelerating the drug research and development process, reducing development costs, and ensuring the stability and consistency of drug molecules, thereby improving drug efficacy and safety. The strength and technological innovation capability of excellent technological platforms lay the foundation for Huahao Zhongtian to continuously and efficiently obtain innovative anti-tumor drugs with high drugability and strong scalability in the "same category first creation" and "same category best".
Relying on the three platforms, Huahao Zhongtian has made rapid progress in the field of anti-tumor new drug research and development. The prospectus shows that in addition to the previously mentioned Utidelong injection, Huahao Zhongtian already has 19 other pipeline products, including new products such as Utidelong capsules, Utidelong nanometer formulations, Utidelong antibody drug conjugates, BG22, BG18, among others.
The turning point of the S-curve may be at hand.
For innovative drugs, especially those targeting new targets and mechanisms, their growth cycles are often discontinuous and leapfrogging because scientific development itself is disruptive. Therefore, it becomes particularly important to innovate in line with product innovation cycles and growth cycles for innovative drug investment.
The growth curve of innovative drugs often takes the form of an S-shaped growth curve. The S-curve is mainly divided into three stages, including the technology introduction period, technology growth period, and technology maturity period.
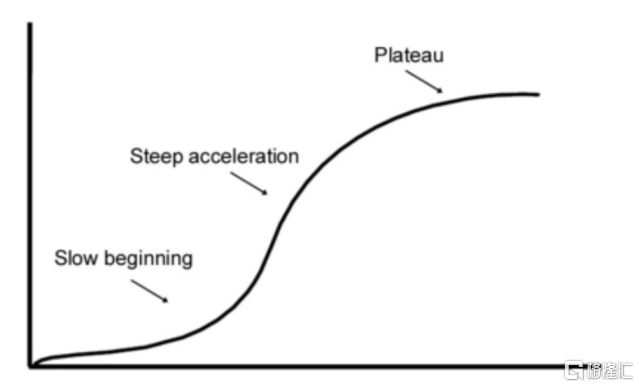
In the initial stage of technology, due to factors such as high R&D costs, high technical difficulty, and low market awareness, corporate progress is usually relatively slow. During this period, companies need to invest a lot of resources in technical research and market promotion, but the returns may not be obvious, and the growth curve is slow. Once the technical breakthrough key bottlenecks are overcome, they enter the technology growth period, and companies will show exponential growth. At this time, market demand rapidly expands, and companies begin to receive significant economic returns. For innovative drugs, once the drug is successfully developed and launched, its sales will increase rapidly and become a new growth engine for the company. With the continuous maturity of technology and market saturation, the growth rate will gradually slow down, and companies will enter the technology maturity period. At this point, companies need to find new growth points to sustain the continuous development of the business.
Huahao Zhongtian may be on the eve of entering the technology growth period. Its core product Yutidelong injection was approved by the National Medical Products Administration in 2021 and was quickly included in the National Medical Insurance Drug List in 2023. However, the sales peak of Yutidelong injection has not yet arrived. Currently, the company's products have been granted access to approximately 509 hospitals and are striving to enter more hospitals. The penetration rate of the product among patients is also increasing.
In addition, according to the company's prospectus, Huahao Zhongtian is also continuously expanding multiple new indications of Yutidelong through clinical trials, such as late-stage non-small cell lung cancer, breast cancer neoadjuvant therapy, late-stage gastric cancer or esophageal cancer, cancer brain metastasis, and many other Phase 2 and Phase 3 China and global international multicenter clinical trials are being conducted simultaneously. The gradual approval of these indications will continue to expand the market space of Yutidelong.
The company is also exploring the development of more dosage forms to meet market demand. Currently, conventional formulations of paclitaxel drugs in China are all injections, which need to be prepared and administered intravenously in hospitals, requiring patients to return to the hospital frequently and may cause adverse reactions at the injection site. Therefore, the development of oral formulations has always been a hot topic in the industry. Huahao Zhongtian is promoting the clinical research of Yutidelong oral capsules in China and the USA concurrently. In August of this year, the Phase I clinical study of Yutidelong oral capsules for advanced solid tumor patients in the USA (NCT05681000) completed the enrollment of all patients and showed promising efficacy and safety data.
From a financial perspective, in the first 5 months of 2022, 2023, and 2024, Huahao Zhongtian's revenue was 32.82 million yuan, 66.635 million yuan, and 28.564 million yuan respectively. The company has transitioned from a high investment phase to a high growth phase.
For Huahao Zhongtian, the current stage is crucial. At this stage, Huahao Zhongtian has surpassed the high-risk start-up phase and is on the eve of rapid growth. Therefore, now may be a good opportunity for investors to pay attention to Huahao Zhongtian.

 笔者关注到,在此背景下,港股迎来“合成生物学医药第一股”
笔者关注到,在此背景下,港股迎来“合成生物学医药第一股”