The core product has been on the market for many years and has not yet escaped from the loss. What's next for Huahao Zhongtian Pharmaceuticals-B (02563)?
In the current resurgence of the pharmaceutical sector, the Hong Kong stock market has also welcomed an innovative drug company with the halo of a U.S. orphan drug - Huahao Zhongtian Pharmaceuticals-B (02563). During the period from October 23 to October 28, Huahao Zhongtian Pharmaceuticals, known as the 'first stock in synthetic biology medicine,' conducted its IPO, with a price range of HK$16 to HK$22 per share, 200 shares per hand, and is expected to be listed on October 31.
Public information shows that based on the median IPO price of HK$19, the company raised approximately HK$0.277 billion, with four cornerstone investors subscribing a total of 23 million U.S. dollars (approximately HK$0.179 billion), accounting for 64.13% of the total shares to be subscribed.
 As of the close on the 28th, the subscription multiple of the stock was 10.42 times, with a relatively tepid market response. If the final public subscription multiple is less than 15 times but initiates an active buyback, there is a high probability of a significant decrease in the stock price after the company goes public. The high upper and lower limits of the IPO price of up to 38% also seem to indicate a certain level of risk.
As of the close on the 28th, the subscription multiple of the stock was 10.42 times, with a relatively tepid market response. If the final public subscription multiple is less than 15 times but initiates an active buyback, there is a high probability of a significant decrease in the stock price after the company goes public. The high upper and lower limits of the IPO price of up to 38% also seem to indicate a certain level of risk.
The core product has been on the market for many years and has not yet escaped from the loss.
Public information shows that Huahao Zhongtian Pharmaceuticals, established in 2002, is a biomedical company driven by synthetic biology technology. It is dedicated to the development of innovative drugs for tumors and has successfully developed three core technology platforms focusing on the research and development of new drugs from microbial metabolites.
As of October 14, 2024, Huahao Zhongtian Pharmaceuticals has one commercialized product and 19 other pipeline candidate products. Its clinical trials and projects cover indications for late-stage breast cancer, advanced non-small cell lung cancer (NSCLC), neoadjuvant therapy for breast cancer, gastric cancer, esophageal cancer, breast cancer brain metastasis, lung cancer brain metastasis, glioblastoma, and other solid tumors.
According to the prospectus, Huahao Zhongtian has not been profitable so far. The company's revenue in 2022 and 2023 was 32.82 million yuan and 66.64 million yuan respectively. It suffered losses of 0.161 billion yuan and 0.19 billion yuan during the period. In the first 5 months of 2024, the company's revenue was 28.56 million yuan, a 5.58% increase compared to the same period last year, with operational losses of 57.43 million yuan, a 31.14% decrease from the same period last year.
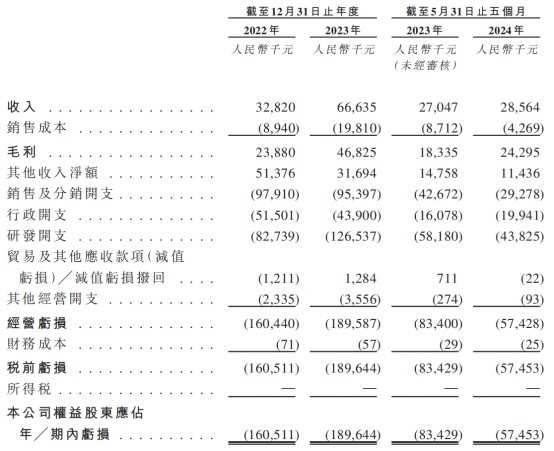
Core products have been on the market for many years but have consistently failed to break even, which is partly related to medical insurance price negotiations. It is understood that the company's core product, Yutaida long-acting injection, was approved for listing by the National Medical Products Administration in 2021 at an average selling price of 2388.71 yuan per bottle. It was included in the National Medical Insurance Drug List in 2022, and on March 1, 2023, the negotiated price officially took effect, resulting in a price drop of over 60% for the product.
However, thanks to the increase in medical insurance coverage, product sales have also increased accordingly. According to the prospectus, from 2021 to May 2024, the sales volume of Yutaida long-acting injection was 29,750 bottles, 18,483 bottles, 90,021 bottles, and 38,577 bottles respectively.
It is understood that sales expenses of the company increased during the reporting period, reaching as high as 0.19 billion yuan at one point. From 2019 to 2021, Huahao Zhongtian's sales expenses were 21.7936 million yuan, 16.1033 million yuan, and 191.7839 million yuan, respectively. From 2022 to May 2024, the company's sales and distribution expenses were 97.91 million yuan, 95.397 million yuan, and 29.278 million yuan, with marketing expenses accounting for 28.8%, 39.6%, and 39.7% of sales and distribution expenses respectively.
During the period, the company had a high concentration of customers, with the top five customers contributing over 80% of revenue. From 2021 to May 2024, sales revenue from the top five customers of Huahao Zhongtian accounted for 84.91%, 81.6%, 88.5%, and 84% of total revenue respectively.
However, despite the recent trend of declining capacity utilization rates, Huahao Zhongtian is planning to continue fundraising for expansion through its IPO, which may bring significant sales pressure to the company in the future.
The IPO prospectus shows that the company plans to use approximately 44.9% of the funds raised to advance clinical trials of core products, about 38.9% for research and experiments beyond core products, about 3.0% to enhance domestic commercialization capabilities and establish a global marketing network, and about 3.2% to expand production capacity.
It is understood that the current production capacity of the first phase production facilities of Huahao Zhongtian can produce 0.5 million bottles of Youtidelong injection per year. The second phase production facilities are expected to be put into operation in 2025, with the total production capacity expected to reach at least 1 million bottles of Youtidelong injection and at least 2 million Youtidelong capsules per year by 2025.
However, according to the prospectus, in recent years, the company's production utilization rate has been less than forty percent, while inventory surplus and inventory turnover days are at a high level. From 2021 to 2023 and from January to May 2024, the utilization rates of the first phase production facilities of Huahao Zhongtian were 13.66%, 5.5%, 39.4%, 0% respectively. During the same period, the company had 20,975 bottles, 107,608 bottles, 88,745 bottles of Youtidelong injections in stock, while the sales volumes during the same period were 18,483 bottles, 90,021 bottles, and 38,577 bottles respectively.
According to the Securities Times APP, as of December 31, 2022 and 2023, and May 31, 2024, the inventory surpluses were approximately 31.1 million yuan, 27.3 million yuan, and 31.2 million yuan respectively; for the fiscal year ending December 31, 2022 and 2023, and the five months ending May 31, 2024, the inventory turnover days were 1,098 days, 538 days, and 1,040 days respectively.
The production capacity utilization rate is clearly not saturated, coupled with the company's single product structure, which may raise market concerns about the company's future profitability.
Fully betting on Youtidelong, will the halo of "orphan drug" fail to mask profit concerns?
From the perspective of product structure and R&D pipeline, currently Huahao Zhongtian mainly focuses on the layout of Youtidelong injections. Among Huahao Zhongtian's core products and 19 candidate products, 16 are all based on the single active pharmaceutical ingredient Youtidelong. There are three formulations of it in the company's product portfolio. As for other candidate products like BG22, BG18, BG44, they are still in the early preclinical development stage.
Public information shows that Youtidelong injections are more advanced in the clinical progress of indications for late-stage non-small cell lung cancer, late-stage breast cancer, and breast cancer neoadjuvant therapy, all of which have reached phase 3 clinical trials.
In recent years, the company's investment in research and development has been continuously increasing. From 2022 to 2023 and from January to May 2024, Huahao Zhongtian's research and development expenses were 82.739 million yuan, 126.537 million yuan, 43.825 million yuan respectively. During the same period, the proportion of core product research and development expenses to total research and development expenses were 61.4%, 77.9%, 70.1% respectively.
It can be seen that Huahao Zhongtian has focused all its efforts on Youtildron, but what is the exact commercial potential of Youtildron?
According to the Zhitong Finance and Economics APP, in 2021, Youtildron injection was approved for market launch, ending the situation of the lack of domestically produced first-class chemotherapeutic innovative drugs with independent research and development in China for nearly twenty years. As of October 14, 2024, Youtildron injection is the only chemotherapeutic drug developed and approved for market launch using synthetic biology technology, and it is the only tubulin inhibitor type of tumor drug with a novel molecular structure approved globally since 2010.
Youtildron has the characteristic of a wide spectrum of anti-cancer activities. Early research results show significant anti-tumor activity against common tumors such as breast cancer, lung cancer, liver cancer, colorectal cancer, and prostate cancer, superior to paclitaxel. It also exhibits good therapeutic effects on multi-drug resistant tumors resistant to paclitaxel and other chemotherapeutic drugs.
In the USA, the company has obtained orphan drug designations for Youtildron injection to treat breast cancer brain metastases, and Youtildron capsules to treat advanced gastric and esophageal cancers from the FDA. According to the Orphan Drug Act enacted in the USA in 1983, drugs that are granted orphan drug designation by the US can enjoy a series of accelerated review and special support policies, including 7 years of market exclusivity after new drug approval, exemption from NDA/BLA application fees, possible exemption from certain clinical data submission requirements, and a 50% tax credit on clinical research expenses.
In recent years, the global oncology drug market has rapidly expanded, from $128.1 billion in 2018 to $228.9 billion in 2023, and is expected to reach $419.8 billion by 2030, with a compound annual growth rate of 9.1% from 2023. Benefiting from factors such as accelerated approval procedures for innovative drugs and reforms in medical insurance payment methods, China's oncology drug market has increased from 157.5 billion yuan in 2018 to 241.6 billion yuan in 2023, and is expected to reach 548.4 billion yuan by 2030, with a compound annual growth rate of 12.4% from 2023.
According to the case data in 2023, breast cancer ranks second among the top ten most common cancers globally, with 2.408 million cases, indicating a vast potential market space.

However, as an orphan drug with a small patient population and low market demand, the market penetration rate of utidelonate injection for advanced breast cancer is relatively limited, and its price reduction by more than 60% after being included in medical insurance has also severely affected profitability.
The weak profit performance and the research and development relying solely on a single core product have resulted in significant uncertainty in the company's future performance. Relying solely on the halo of anti-cancer orphan drugs, Hua Hao Zhong Tian may not be convincing enough to persuade investors to pay with real gold and silver.

 截至28日收盘,该股融资申购倍数为10.42倍,市场反应较为冷淡,若最终公开认购倍数不足15倍却开启主动回拨,公司上市后股价有较大概率下跌。而公司招股价上下限幅度高达38%,也似乎表明存在一定风险。
截至28日收盘,该股融资申购倍数为10.42倍,市场反应较为冷淡,若最终公开认购倍数不足15倍却开启主动回拨,公司上市后股价有较大概率下跌。而公司招股价上下限幅度高达38%,也似乎表明存在一定风险。