For domestic innovative drug companies, the medical insurance catalog has now become a "gateway." As long as they successfully pass through this gateway, innovative drug enterprises can rely on the logic of exchanging price for quantity to achieve rapid production increase, thereby stepping up the company's cashing out speed and hematopoietic capacity.
Since the normalization of the domestic medical insurance negotiation mechanism in 2017, 7 batches of negotiations have been completed between 2017 and 2023. From October 16 to 18, with the smooth conduct of pre-negotiations for medical insurance, it means that the national medical insurance formal negotiations for this year are about to begin.
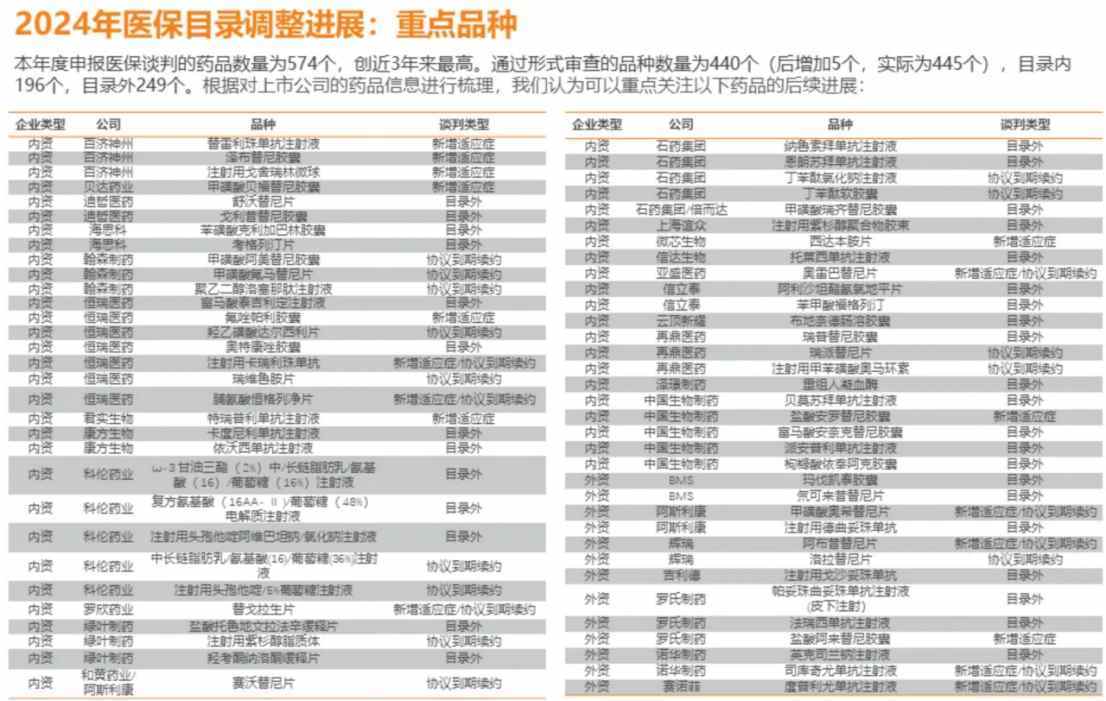
Data shows that the number of drugs applying for medical insurance negotiations this year is 574, the highest in nearly 3 years. A total of 445 drugs have passed the formal review, with 249 outside the catalog and 196 within the catalog. Compared to the situation last year where 570 drugs and 390 passed the initial formal review, the number of drugs applying for and passing the review has increased. The negotiation performance and results of innovative drugs continue to be a focus of the industry.
"Protecting the basic" positioning remains unchanged, with increased support for innovation.
 The timing, process, and flow of this year's medical insurance negotiations are basically the same as past medical insurance catalog adjustments, still divided into five stages: preparation, application, expert review, negotiation, and result publication.
The timing, process, and flow of this year's medical insurance negotiations are basically the same as past medical insurance catalog adjustments, still divided into five stages: preparation, application, expert review, negotiation, and result publication.
In September this year, the National Medical Insurance Administration has organized experts to review the application drugs for the formal review of the adjustments to the national medical insurance catalog in 2024 and completed the review. According to the normalization work timeline, the next steps will enter the negotiation / bidding phase (September to November 2024) and the result publication phase (November 2024).
It is worth mentioning that while sticking to the normalization work, the national medical insurance drug catalog access and exit mechanism is constantly being improved. Recently, documents such as "2024 National Basic Medical Insurance, Work Injury Insurance, and Maternity Insurance Drug Catalog Adjustment Work Plan (Draft for Soliciting Opinions)" and "2024 National Basic Medical Insurance, Work Injury Insurance, and Maternity Insurance Drug Catalog Adjustment Application Guide (Draft for Soliciting Opinions)" have been released.
Overall, the renewal and bidding procedures remain the same as last year, with the addition of a rule for a category of drugs to be removed, such as regular catalog drugs that have not been supplied to designated medical institutions by medical insurance in the past 3 years; drugs under negotiation that have not ensured market supply as agreed by June 30, 2024. Looking at the direction of reform, the overall theme of medical insurance catalog adjustments still adheres to the positioning of "protecting the basic," accelerating the continuous release of innovative energy based on this foundation.
In recent years, it is widely recognized that the country has shown strong support for the innovative drug industry in terms of policies, and the annual medical insurance negotiations have gradually become one of the key focuses for domestic innovative drug enterprises.
From a policy perspective, for example, in the recent review of the medical insurance drug list, there has been a policy emphasis on prioritizing the inclusion of innovative drugs in the catalog, and transforming the innovative advantages of drugs into access advantages based on the level of innovation and clinical benefits of the drugs.
For innovative drugs listed in the catalog, the National Healthcare Security Administration introduced a simple renewal mechanism as early as 2022. According to the current regulations, as long as the actual fund expenditure for a drug listed in the medical insurance catalog does not exceed 200% of the budget, it qualifies for a simple renewal. Moreover, for innovative drugs triggering a simple renewal, the policy allows companies to apply for renegotiation, where the reduction determined through renegotiation can be smaller than the reduction through simple renewal, thereby benefiting more innovative drugs. This year, in the review process, the number of varieties in the form review of the catalog reached a historical high of 196, reflecting the industry's recognition and positive response to the policies.
As for drugs outside the catalog, the approval rate this year is only 43.4%. Data shows that in the years 2020 to 2023, the approval rates in the expert review process were 42.9%, 43.2%, 45.6%, and 43.5%, all below 50%. This year, the entry approval rate is expected to be once again below 50%.
In fact, the main goal of expert reviews is to include unique drugs with high clinical value, high innovation, and affordability in the national medical insurance negotiation list, preventing pharmaceutical companies lacking clinical value from diluting the representation of true innovation in listed innovative drug companies in China. Therefore, the requirements for expert reviews are high, serving as a specific way to further support genuine innovation in the country.
The Hong Kong stock market's innovative drug sector has seen a clear turning point.
For domestic innovative drug enterprises, the medical insurance catalog has now become a 'threshold'. As long as the innovative drug enterprises successfully cross the threshold, they can rely on the logic of trading price for quantity to achieve rapid volume expansion of products, thereby crossing a major step in the company's cash realization speed and hematopoietic ability.
Investment logic still focuses on the commercialization and hematopoiesis of pharmaceutical companies in the Hong Kong stock market's innovative drug sector. "Inclusion in medical insurance" has even become one of the key factors driving valuation growth for innovative drug companies, accelerating the formation of a turning point in the Hong Kong stock market's innovative drug sector.
Hong Kong's 18A policy has provided many unprofitable innovative drug companies with the opportunity for public financing, but since the global biomedical financing winter in 2021, investors' investment logic in the innovative drug sector has become more conservative.
Data shows that in 2023, the financing amount of innovative drugs in China in the primary and secondary markets has fallen back to the level of 2018, which is 30.9 billion yuan and 21.5 billion yuan respectively. At its peak in 2021, this number was 88.8 billion yuan. Internally, the long research and development cycle, uncertainties, and the inability to prove growth logic with product profit are the main reasons why innovative drug companies have become the primary targets of short selling for investors.
Although medical insurance negotiations cannot solve the efficiency issues in pharmaceutical research and development, they can address market problems for pharmaceutical companies with innovative products ready for commercialization.
Previous data shows that with the stabilization of medical insurance negotiation rules, the success rate of medical insurance negotiations from 2019 to 2023 has been on the rise, especially the significantly increased possibility of innovative drugs being included in the medical insurance catalog. In terms of the 2023 negotiation drug list, 25 innovative drugs participated in the negotiations, with 23 successful negotiations, achieving a high success rate of 92%.
In terms of trading price for volume, data shows that before the implementation of medical insurance, pharmaceutical sales volume and revenue growth were relatively slow. Starting from the year of medical insurance implementation, the sales volume showed a year-on-year growth of about 50 times, while the revenue showed a growth of about 10 times. According to research report data from Southwest Securities, the average price reduction of newly added varieties in medical insurance negotiations from 2019 to 2022 was 61%, 51%, 62%; the average sales revenue increase in the first year of medical insurance reached 2692%, 5697%, 15367% respectively.
Furthermore, comparing the sales of new products under medical insurance and non-medical insurance from their launch until the end of 2023, it can be observed that nearly 2/3 of the sales revenue of medical insurance drugs accumulates to over a billion yuan, with approximately 50% of products having sales revenue exceeding 0.5 billion yuan, 21.7% exceeding 1 billion yuan, and several products having sales revenue exceeding hundreds of billions yuan. In contrast, among non-medical insurance products, 78.3% of products have not achieved sales revenue of over a billion yuan by the end of 2023, with only 6.7% having sales revenue exceeding 1 billion yuan, considerably lower than the proportion of medical insurance products exceeding 1 billion.
Returning to the Hong Kong innovative drug market, in the 2024 national negotiations, Hong Kong-listed companies, and key varieties include Beigene: Tislelizumab (new indication), Zepzelca (new indication), Gavreto (new indication); Junshi Bio: Toripalimab (new indication); Sino Biopharm: Bevacizumab (first time), Anlotinib (new indication), Anlotinib (first time); Akeso: Camrelizumab (first time), Envafolimab (first time); Luye Pharma: Toripalimab Slow-release Tablets (first time), Hydrocodone/Naloxone Slow-release Tablets (first time), Paclitaxel Liposomes (renewal); CSPC Pharma: Narulose Bevacizumab (first time), Olaratumab (first time), Diclofenac Sodium Injection (renewal), Diclofenac Soft Capsules (renewal); Hansoh Pharma: Amevatinib (renewal), Flumatinib (renewal), Pegloticase (renewal); HUTCHMED (China): Savolitinib (renewal).
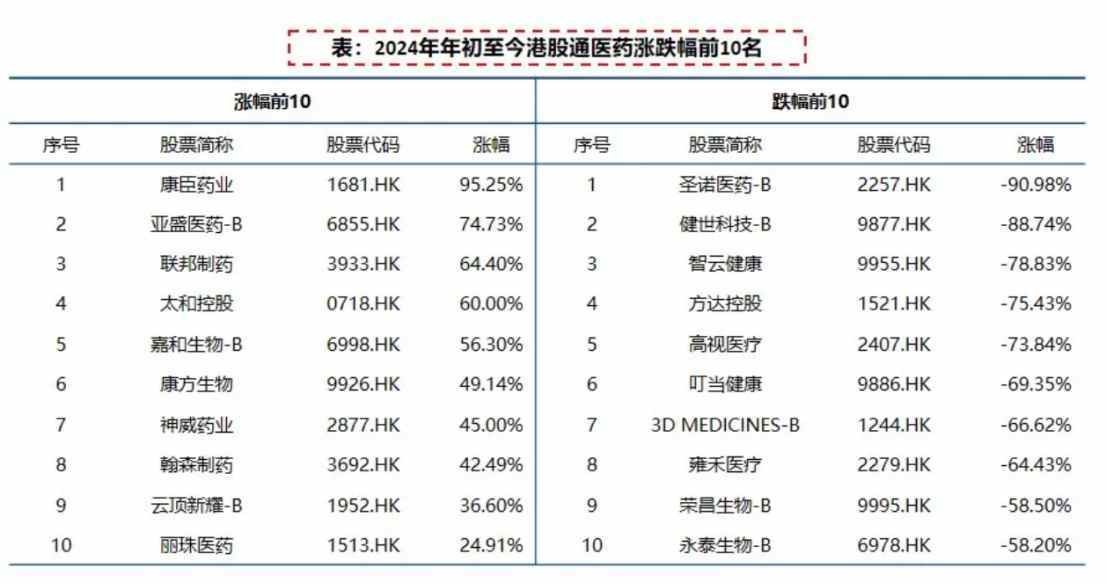
In terms of secondary market performance, the top ten pharmaceutical companies under the Hong Kong Stock Connect in the pharmaceutical sector from the beginning of this year to now are almost all innovative drug companies that have realized product commercialization scale and have core products included in the medical insurance catalog, with the turning point in the rate of increase becoming more evident.
Editor/rice

 今年医保谈判的时间节奏、流程与过往医保目录调整基本一致,依旧分为准备、申报、专家评审、谈判和公布结果五个阶段。
今年医保谈判的时间节奏、流程与过往医保目录调整基本一致,依旧分为准备、申报、专家评审、谈判和公布结果五个阶段。