①Yinuosi's original plan was to raise 1.602 billion yuan, but only raised 0.672 billion yuan. As a result, it had to significantly reduce the investment amount of some of the raised projects and also canceled the flow compensation project. ②Due to the slowdown in investment from the capital market, the growth rate of China's pharmaceutical R&D expenses has also started to slow down, and many upstream CRO companies, including Yinuosi, have been impacted.
On October 16, the Science and Technology Innovation Board Daily (Reporter Zheng Bingxun) reported that in early September this year, Yinuosi (688710.SH) successfully listed on the A-share market with the dual halo of being the "first CRO stock owned by a central enterprise" and the "first pharmaceutical company to land on the Science and Technology Innovation Board". It quickly became the focus of the market.
However, upon closer examination after the initial hype, Yinuosi's initial fundraising plan of 1.602 billion yuan fell significantly short, only raising 0.672 billion yuan, a reduction of over 58%. After deducting various expenses, the actual net amount raised was 0.61 billion yuan. This to some extent reflects the current 'temperature' of the market.
 Affected by this, Yinuosi recently passed the "Proposal on Adjusting the Planned Investment Amount of the Raised Funds for the Raised Investing Projects", and based on the actual net amount raised, it adjusted the fund allocation for the investing projects downward.
Affected by this, Yinuosi recently passed the "Proposal on Adjusting the Planned Investment Amount of the Raised Funds for the Raised Investing Projects", and based on the actual net amount raised, it adjusted the fund allocation for the investing projects downward.
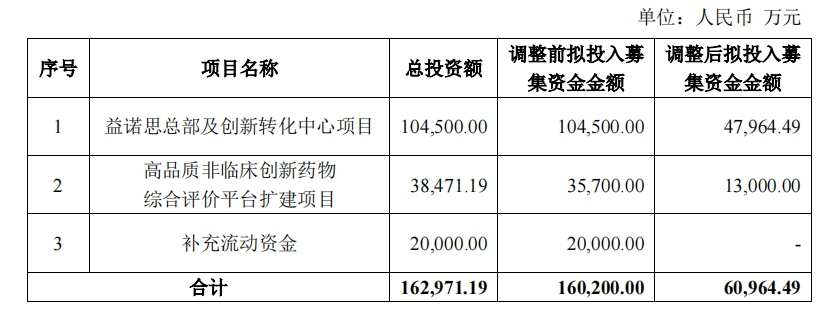
▌Yinuosi significantly reduces the amount allocated to investing projects
The IPO documents show that Yinuosi originally planned to use the raised 1.602 billion yuan for 3 projects, including the Yinuosi headquarters and Innovation Transformation Center project (referred to as the 'headquarters project'), the comprehensive evaluation platform expansion project for high-quality non-clinical innovative drugs (referred to as the 'platform project'), and for working capital replenishment. Among them, the headquarters project was planned to receive 1.045 billion yuan of the raised funds, accounting for 65.23%, the platform project was planned to receive 0.357 billion yuan, accounting for 22.28%, and the remaining 0.2 billion yuan was to be used for flow compensation.
According to the adjusted plan, the latest planned investment for the headquarters project is 0.48 billion yuan, a reduction of 54.10% from the original, and the latest planned investment for the platform project is 0.13 billion yuan, a reduction of 63.59%. The original flow compensation project has been directly canceled.
According to the initial plan, the implementation of the headquarters project is to alleviate the problem of insufficient space at Innoscience Lab. The total land area of the project is approximately 16437.7 square meters, with a planned total construction area of 77812.7 square meters, intending to build a research headquarters building, safety assessment laboratory, animal facilities building, clinical analysis and translational center, and basement.
The platform project is mainly aimed at solving the inadequate animal facilities for conducting CRO business. Innoscience originally planned to purchase a factory in Nantong with an area of 24115.93 square meters, including the renovation of animal rooms, laboratories, and other facilities.
Innoscience stated that this adjustment is made to ensure the smooth implementation of the investment projects and the efficient use of raised funds, aligning with the company's future global strategy. For the insufficient raised funds, Innoscience will address it using its own funds. Wind data shows that as of the first half of 2024, Innoscience had cash and cash equivalents balance of 0.988 billion yuan.
Innovative drugs 'icebreaker,' CRO company's foresight.
Innoscience's fundraising falling short of expectations is a common reflection of the cooling trend in the CRO industry. This can be evidenced by the performance decline of Innoscience and several other CRO companies in the industry.
Data shows that Innoscience is a CRO company providing services in non-clinical research and clinical testing. Non-clinical CRO business is Innoscience's core source of income, consistently accounting for over 90% of total revenue. In 2023, the share of revenue from non-clinical CRO business reached as high as 96.25%.
Furthermore, within the non-clinical CRO business, 'safety evaluation' is of utmost importance for Innoscience. In 2021 and 2022, Innoscience's non-clinical safety evaluation business achieved revenues of 0.29 billion yuan and 0.414 billion yuan respectively, accounting for 83.96% and 82.31% of total revenue. According to Frost & Sullivan statistics, Innoscience secured a market share of 6.10% and 6.80% in the field of non-clinical safety evaluation in 2021 and 2022 respectively, ranking third in the domestic market and holding a leading position in the industry.
Furthermore, between 2021 and 2023, over 90% of Innoscience's annual revenue came from non-clinical research services for Class I innovative drugs, with its main customers including Jiangsu Hengrui Pharmaceuticals, CSPC Pharma, BaiLi Pharmaceuticals, Qilu Pharmaceutical, Junshi Bio, and other companies.
However, due to factors such as the weakening of investment and financing enthusiasm, the slower-than-expected growth of the pharmaceutical market, the demand growth rate of the CRO industry has slowed down. At the same time, compounded by intensified industry competition, the decrease in prices of experimental monkeys, and other factors, the price of new contracts signed by Joinn falls since the second half of 2023, leading to a decline in overall profitability.
In the first half of 2024, Joinn achieved a revenue of 0.606 billion yuan, a year-on-year increase of 15.01%, with a net profit attributable to the parent company of 95.6391 million yuan, a year-on-year decrease of 16.05%. At the same time, the gross margin rate in the first half of 2024 decreased by 8.23 percentage points year-on-year.
In fact, Joinn's poor performance, is also being played out in other CRO companies within the industry.
Shanghai Medicilon Inc. (688202.SH) revealed that due to intensified competition in the pharmaceutical industry, declining order prices, and some delayed executions of orders, revenue in the first half of 2024 decreased by 40.23% to 0.521 billion yuan, with a net loss attributable to the parent company of 70.2303 million yuan, a year-on-year decrease of 142.27%. Meanwhile, Joinn Laboratories (603127.SH) achieved a revenue of 0.849 billion yuan in the first half of 2024, a year-on-year decrease of 16.08%, with a net loss attributable to the parent company of 0.17 billion yuan, a year-on-year decrease of 287.30%.
According to Frost & Sullivan data, affected by the slowdown in domestic innovative drug financing, China's pharmaceutical R&D expenditure growth rate witnessed a cliff-like decline in 2022, with a total R&D expenditure of 210.8 billion yuan for the year, a year-on-year increase of 2.48%, which was more than 18 percentage points lower than the growth rate of 20.79% in 2021. It is expected that in the coming years, China's pharmaceutical R&D expenditure will continue to grow, but the growth rate is significantly lower than the high level. The total R&D expenditure is expected to be 247.6 billion yuan in 2024, a year-on-year increase of 12.65%.
Against this backdrop, Joinn Laboratories predicts that the non-controlling net profit attributable to the parent company from January to September 2024 is estimated to be around 0.119 billion to 0.13 billion yuan, a year-on-year decrease of 21.93% to 28.53%, with a decrease exceeding 20%. Looking ahead to 2024, due to the continued impact of declining order prices, the full-year non-controlling net profit attributable to the parent company is expected to decline by up to 20% compared to 2023.

 受此影响,益诺思于近日通过《关于调整募投项目拟投入募集资金金额的议案》,根据实际募资净额,对募投项目的资金安排进行调降。
受此影响,益诺思于近日通过《关于调整募投项目拟投入募集资金金额的议案》,根据实际募资净额,对募投项目的资金安排进行调降。