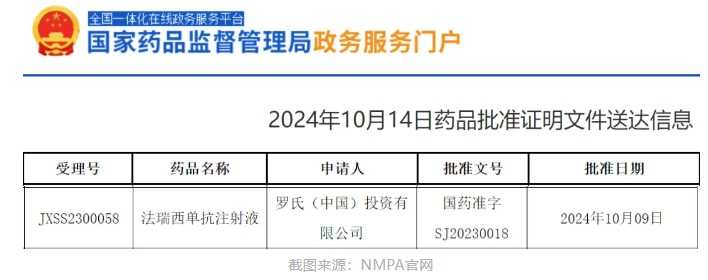
The latest announcement on the official website of the China National Medical Products Administration (NMPA) shows that Roche (RHHBY.US) Faricimab injection's new indication has been approved for market application.
According to the latest announcement on the official website of the China National Medical Products Administration (NMPA) on October 14th, Roche (RHHBY.US) Faricimab injection's new indication has been approved for market application. According to a previous Roche press release, this is the fourth approved indication for Faricimab in China, used to treat macular edema secondary to central retinal vein occlusion (CRVO).
It is reported that retinal vein occlusion (RVO) is a retinal vascular disease caused by various factors leading to partial or complete blockage of retinal veins, resulting in impaired blood flow in the retinal vein system, and is manifested as retinal vascular diseases such as retinal vein dilation and retinal hemorrhage. Depending on the location of the vascular occlusion, it can be divided into branch retinal vein occlusion (BRVO) and central retinal vein occlusion (CRVO).