Recently, in addition to the Fed cutting interest rates to inject liquidity into the market, the unexpected adjustment of domestic monetary policy has fully stimulated the vitality of the capital markets.
The heavy policies and voices from the three financial departments, the Political Bureau meetings, the People's Bank of China, and others have caused both A-shares and Hong Kong stocks to continue to rise like ignited firecrackers.
According to Futubull data, in the past month, the gains of several indexes in A shares have reached historic highs, with the total trading volume of the three cities in the Shanghai, Shenzhen, and Beijing regions exceeding the 3 trillion yuan mark for the first time. The Hang Seng Index and the Hang Seng Tech Index in Hong Kong have both reached their highest levels since February 2022.
 Among them, the innovative drug sector, due to its long-term valuation bottoming out and high growth potential, has a high cost-effectiveness in terms of investment, making it one of the important directions worth paying attention to in this round of the market.
Among them, the innovative drug sector, due to its long-term valuation bottoming out and high growth potential, has a high cost-effectiveness in terms of investment, making it one of the important directions worth paying attention to in this round of the market.
Capital also seems to have reached a consensus on this, successively voting for the innovative drug sector.
According to Futubull data, in the past month, the onshore trading volume of the highest purity Hong Kong Connect Innovative Drugs ETF (159570.SZ) has been active, with gains exceeding 30% at one point. As of now, the trading volume has exceeded 0.2 billion yuan for four consecutive trading days, with net inflows of funds exceeding 0.257 billion yuan in the past five trading days. The trend of fund inflows further confirms the attractiveness of the innovative drug sector.
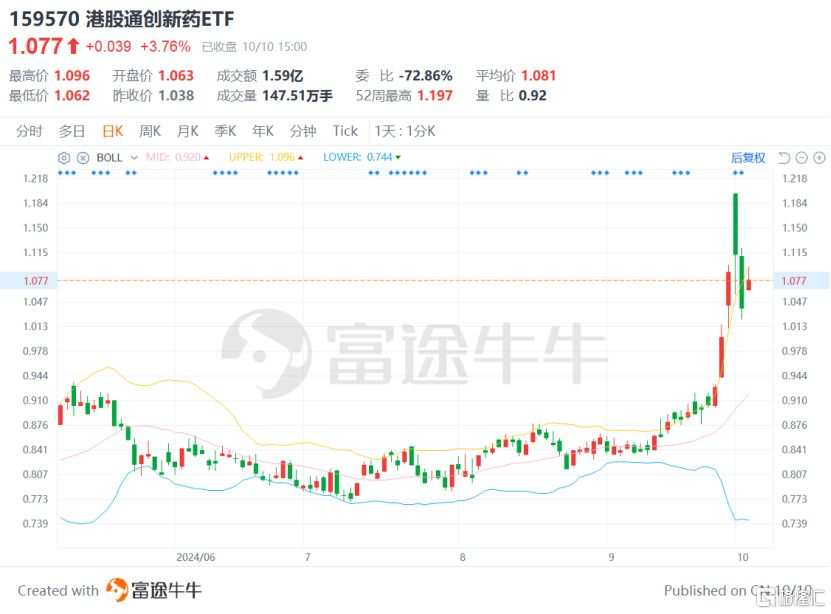
(Source: Futubull)
Unlike many innovative drug companies that do not yet have commercialized products, one enterprise has crossed the threshold of commercialization and achieved profitability at the commercial level for the first time in the first half of this year, that is Yunding Xinyao.
From the perspective of the capital market performance, in the past two months, Yunding Xinyao's stock price has once risen by more than 60%, indicating that its internal growth momentum has just begun.
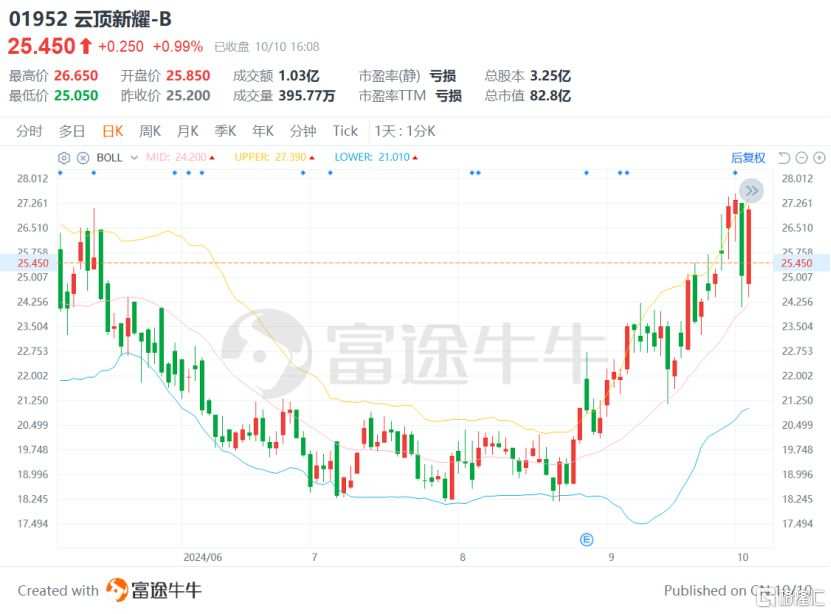
(Source: Futubull)
In order to further verify the future growth potential of Yunding Xinyao, we may focus on two dimensions: short-term and long-term, to further discuss: the company's current fundamentals and future growth certainty.
1. Short-term: Global innovative pipeline precise layout, gradually realizing value
As an innovative drug company focusing on innovative drugs and vaccines, Yunding Xinyao has established a global strategic vision from the beginning, focusing on the Asia-Pacific market, dedicated to addressing global unmet medical needs, and maximizing the commercial value of its products.
The company has established a strong product pipeline in the areas of kidney, anti-infection, and autoimmune diseases, with some products holding global commercial rights, covering multiple potential first-in-class or best-in-class products.
Taking two commercialized products as examples, Nifekang is the world's first innovative drug for IgA nephropathy; while Elacom (Eleranse) is the world's first fluorine-class antibiotic, also the company's first commercialized product in China.
These two globally pioneering products were introduced from overseas by Cloud Peak in just five years and were approved for listing domestically. This not only reflects the company's profound insights and high clinical strength in the entire innovative drug market, but also demonstrates its leading position and competitiveness in the industry.
There is also a potential best-in-class product in the clinical stage - EVER001, as a global BTK inhibitor with global rights. Different from most covalent irreversible BTK inhibitors worldwide, EVER001 takes a covalent reversible approach, which can maintain high efficiency while being highly selective. It has lower on-target and off-target toxicities. Preliminary Phase Ib clinical results for primary glomerulonephritis are expected to be announced in the second half of this year.
It is worth noting that through Cloud Peak's indication layout, its differentiation focuses on blue ocean areas, demonstrating its ability to identify treatment needs in disease markets with blank spaces.
For example, IgA nephropathy targeted by Nifekang is the most common primary glomerular disease globally. IgA nephropathy has regional and racial differences. Zhang Hong, Director of the Nephrology Department of Peking University First Hospital, pointed out that compared to Europeans and Americans, the disease progression of IgA nephropathy patients in China is faster with a worse prognosis. Data shows that there are 5 million potential IgA nephropathy patients in China, with 0.1 million new diagnosed cases each year, and millions of confirmed IgA nephropathy patients already.
For example, the primary membranous nephropathy targeted by EVER001 is an autoimmune glomerular disease which has seen an increasing incidence in recent years, surpassing IgA nephropathy in some regions. It is one of the main causes of end-stage kidney disease and renal failure in China. Market data shows that the number of patients with primary membranous nephropathy in China is approximately 2 million, significantly exceeding the global total. Research also indicates that about 10% to 40% of primary membranous nephropathy patients may develop end-stage renal disease within 10 to 15 years or longer.
However, the current treatment methods have significant limitations, with large side effects and over 30% of patients showing no response to existing standard therapies. Even among those who have achieved remission, there is a 30% chance of relapse. Therefore, if EVER001 successfully enters commercialization in the future, it will face significant market opportunities.
At the same time, Cloud Peak will continue to enter its harvest period.
In addition to Nifekang and Yijia, the company's products in the commercialization pipeline also include Iquimod, which has been approved for listing in China, Macau, and Singapore, mainly for the treatment of ulcerative colitis. The industry predicts that the peak sales of Iquimod are expected to reach 2 billion yuan. There is also the soon-to-be-launched potent antibiotic Cephalosporin/Taniborbital, with an estimated peak sales of 1.5 billion yuan.

(Source: Company Financial Report)
It can be said that it is this forward-looking global innovative pipeline layout that gives Yunding Xinyao a solid foundation for evolving into Biopharma.
Long-term: "Self-research + BD" dual-wheel drive, stimulating long-term growth potential.
In the innovative drug industry, while the richness of the product pipeline is important, it is only the cornerstone of the long-term development of the enterprise. In order to sustain growth, innovative drug companies must have innovation capabilities and constantly launch new products to provide continuous momentum for the company's future development.
Many investors' understanding of Yunding Xinyao may still be at the stage of its early rapid construction of the product pipeline through the license-in model.
However, as the company realizes that independent research and development is one of the core drivers supporting its long-term growth, it has not only successfully localized the clinical validation of the mRNA platform but also established a complete set of internal end-to-end capabilities, enabling the company to independently develop and manufacture mRNA tumor vaccines with global rights.
Among them, the highlight is Yunding Xinyao's first completely self-developed mRNA tumor vaccine, EVM16.
The vaccine initiated by researchers started the Investigator-Initiated Trial (ITT) in August this year, marking an important step in the mRNA tumor vaccine field. In addition, the company is also advancing the research and development of other tumor vaccines such as tumor-associated antigens (TAA) vaccines, immune-regulating tumor vaccines, and carrying out mRNA-based in vivo CAR-T projects, which are expected to open up new pathways for the treatment of tumors and autoimmune diseases.
Therefore, focus on the judgment of the company's future performance growth.
Yunding Xinyao adopts a dual-driven strategy, continuously introducing new products with high synergy with the company's existing commercial platform on one hand, and the mRNA platform will continue to develop products with global rights. While enhancing pipeline depth, it also creates space for the company's value-added and future external cooperation, achieving steady growth in revenue, operational efficiency, and profits.
From the latest financial report data, relying on Rapid commercialization of two innovative drugs, Naifukang and Yijia, the company achieved revenue of approximately 30.2 billion yuan in the first half of this year, a 158% increase from the second half of 2023. At the same time, through refined management, it achieved an increase in operational efficiency and a significant narrowing of losses, with operating expenses in the first half of the year significantly down by 249%, non-IFRS losses significantly narrowed by 35%, and continuous optimization of financial structure.
In response, Yunding Xinyao's CEO Luo Yongqing is optimistic, expecting that by the end of 2024, three products will achieve commercial listing, reaching a sales target of 7 billion yuan for the whole year, and striving to achieve cash breakeven by the end of 2025.
Conclusion
Whether from a short-term or long-term investment perspective, Yunding Xinyao's growth has strong certainty.
In the short term, the rapid sales growth of Niferex and Yijia directly contributes to the company's revenue growth. Meanwhile, Iquimod and Cefpodoxime/Taniborbactam are also expected to be approved and launched in the next two years, gradually contributing to the company's revenue. In the long term, the pipeline of mRNA tumor vaccine platform continues to be validated, indicating that the company is poised for a new phase of growth.
Of course, apart from the positive impact of the current macroeconomic trends, the policies and market trends in the field of innovative drugs also bring higher certainty to Yunding New Yao's performance in the capital markets in the future.
On October 12, which was just this past Saturday, the Ministry of Finance emphasized the importance of increasing the countercyclical adjustment of fiscal policies and promoting high-quality economic development at a policy briefing held by the State Council Information Office. In response to this, Tianfeng Securities' research report pointed out that this is almost the maximum policy support that the Ministry of Finance can provide within its authority. The most important aspect is not the specific scale of tens of trillions, but the signal that it is transitioning from contractionary fiscal policy to expansionary fiscal policy.
Tianfeng Securities believes that issuing national bonds involves budget adjustments, which must be approved by the Standing Committee of the National People's Congress; otherwise, it will violate legal procedures. The Ministry of Finance's proactive attitude in giving advance notice and hints before the process is completed is significant. In terms of the amount, the fiscal deficit last year was 798.1 billion yuan, with 500 billion yuan of the 1 trillion yuan national bonds issued last year used, accounting for approximately 63% of last year's fiscal deficit. Extrapolating from the same proportion, this year's fiscal deficit is around 3 trillion, and it is estimated that about 2 trillion yuan of national bonds may need to be issued this year.
In terms of policies, this year's Government Work Report emphasizes the importance of accelerating the development of the innovative drug industry and the 'Deepening Medical and Health System Reform Key Tasks for 2024' outlines the guiding ideologies for deeper reform and innovation in the pharmaceutical field. Furthermore, the 'Full Chain Support Implementation Plan for Innovative Drug Development' further clarifies the full chain policy support from research and development to commercialization. The release of a series of policies provides strong support for the breakthrough development of innovative drugs.
In the market aspect, in the first half of 2024, the National Medical Products Administration approved 44 new drugs for initial marketing in China, including 2 Class 1 innovative drugs, indicating that the number of domestically developed innovative drugs is expected to reach a new high. The next two years will be a phase where innovative drug product cycles realize concentrated revenue, and domestically developed innovative drugs are expected to seize the opportunity of a new industry cycle.
Considering the broad development prospects of the industry, the advantages of Yunding New Yao product pipeline, and the potential for future growth, the company's valuation core may be further enhanced.

