Astrazeneca (AZN.US) has had its application for new indications for its antibody drug accepted for review in China.
According to the caijing.com app, on October 11th, China's National Medical Products Administration's Drug Evaluation Center (CDE) website has just announced that the application for the listing of Ravulizumab, submitted by Astrazeneca (AZN.US) and its subsidiary Alexion, focusing on rare diseases, has been accepted for review. Public information shows that Ravulizumab is a long-acting complement C5 protein inhibitor developed by Alexion. It can be found through the CDE website that the first application for the listing of Ravulizumab in China was accepted in December 2023, making this the second listing application for the product in China.
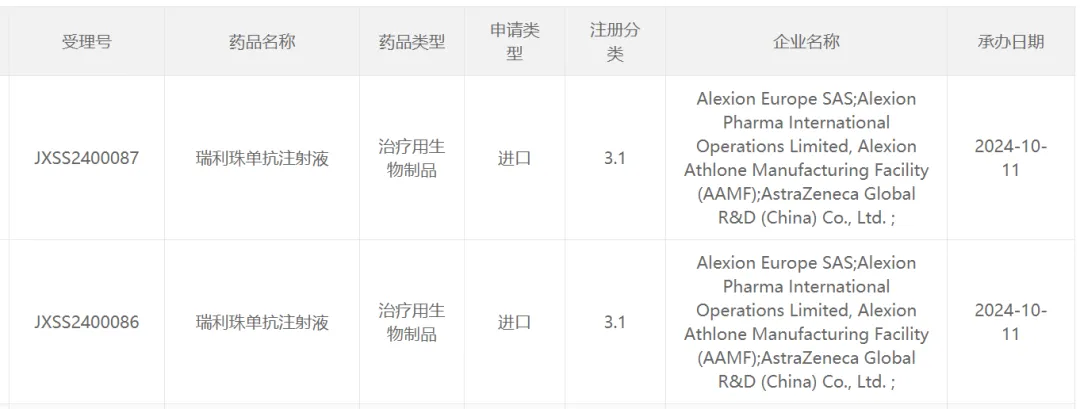
Screenshot source: CDE official website
 Ravulizumab was first approved by the FDA in the usa for market in 2018, used to treat paroxysmal nocturnal hemoglobinuria (PNH), where patients only need an injection every 8 weeks to effectively control hemolysis. Subsequently, the drug has been approved by the FDA to treat various autoimmune diseases, including atypical hemolytic uremic syndrome, myasthenia gravis, neuromyelitis optica spectrum disorder (NMOSD), and more.
Ravulizumab was first approved by the FDA in the usa for market in 2018, used to treat paroxysmal nocturnal hemoglobinuria (PNH), where patients only need an injection every 8 weeks to effectively control hemolysis. Subsequently, the drug has been approved by the FDA to treat various autoimmune diseases, including atypical hemolytic uremic syndrome, myasthenia gravis, neuromyelitis optica spectrum disorder (NMOSD), and more.

 瑞利珠单抗最早于2018年获得美国FDA批准上市,用于治疗阵发性睡眠性血红蛋白尿症(PNH),患者只需要每8周接受一次注射就可以有效控制溶血的发生。此后,该药相继获FDA批准治疗多种自身免疫性疾病,包括非典型溶血性尿毒症综合征、重症肌无力、视神经脊髓炎谱系疾病(NMOSD)等。
瑞利珠单抗最早于2018年获得美国FDA批准上市,用于治疗阵发性睡眠性血红蛋白尿症(PNH),患者只需要每8周接受一次注射就可以有效控制溶血的发生。此后,该药相继获FDA批准治疗多种自身免疫性疾病,包括非典型溶血性尿毒症综合征、重症肌无力、视神经脊髓炎谱系疾病(NMOSD)等。