为了让K药价值持续放大,默沙东开启了一场全球ADC寻觅之旅。
凭借25 billion美元的销售额, $Merck & Co (MRK.US)$ K药成功取代修美乐,登顶2023年“药王”宝座。
整个2023年,默沙东总营收为60.1 billion美元,仅K药这一款以就占据公司总营收的41.6%。对于默沙东而言,延续增长的方法很简单就是持续释放K药的价值,这也是其当下发展的核心战略。
 基于此,默沙东不断帮助K药获批新适应症,今年6月其已经获批了第40个适应症。但即使如此,K药也不是万能的,仍在一些癌种应答率不高,因此默沙东也在不断探索K药联合疗法,如与仑伐替尼联用组成的“可乐组合”,取得了有史以来肝癌治疗的最好效果。
基于此,默沙东不断帮助K药获批新适应症,今年6月其已经获批了第40个适应症。但即使如此,K药也不是万能的,仍在一些癌种应答率不高,因此默沙东也在不断探索K药联合疗法,如与仑伐替尼联用组成的“可乐组合”,取得了有史以来肝癌治疗的最好效果。
寻找“拍档”的过程中,默沙东看中了ADC(抗体药物偶联物)药物的潜力,兼具“靶向性强”和“毒素活性高”这两个优点,如果将K药与ADC联用,有望实现比与化疗、靶向疗法联用更好的效果。
为了让K药价值持续放大,默沙东开启了一场全球ADC寻觅之旅。
01 Regretting Seagen, Kelun Botai fortuitously rises to the top
Among the various ADC companies, Merck recognized the global ADC pioneer Seagen first.
Seagen is truly an ADC veteran, the first ADC drug Adcetris to achieve commercial success globally was funded by Seagen (the first ADC drug Mylotarg was withdrawn from the market due to its toxicity), accumulating over 20 years in the ADC field. If Merck can acquire Seagen, it would be equivalent to swiftly entering the ADC field, thereby forming a strong combination of PD-1 and ADC.
Just as Merck and Seagen were negotiating prices, the 'new crown nouveau riche'$Pfizer (PFE.US)$suddenly emerged, directly acquiring Seagen at a sky-high price of 430 USD, leaving Merck to regretfully withdraw.
After the unsuccessful attempt to acquire Seagen, Merck did not give up on the concept of PD-1 plus ADC. Instead, it turned to search for innovative ADC companies that have not yet been discovered by the market. $SKB BIO-B (06990.HK)$ Being chosen by the goddess of luck like this.
The origin of Sichuan Kelun Pharmaceutical can be traced back to 2012, when the strictest antimicrobial management measures in history, the "Clinical Application Management Measures for Antimicrobial Drugs", were introduced, as the domestic "antibiotic leader".$Sichuan Kelun Pharmaceutical (002422.SZ)$Deeply influenced by policies, the performance of Sichuan Kelun Pharmaceutical has been stagnant. In order to find a breakthrough opportunity, Sichuan Kelun Pharmaceutical proposed the "Three-Driven" strategy, that is, the simultaneous advancement of the three clues of "large infusion, antibiotics, and R&D innovation". Eventually, these three clues developed into Sichuan Kelun Pharmaceutical,$Yili Chuanning Biotechnology (301301.SZ)$, and Sichuan Kelun Botai three companies.
Among them, Sichuan Kelun Botai is the most important offspring of Sichuan Kelun Pharmaceutical in the field of innovative drugs in 2016. Sichuan Kelun Botai proactively targeted the ADC opportunity and launched clinical research on multiple projects such as A167, A166, A140, and SKB264 in the following three years. Relying on the independently developed ADC platform, they have mastered two sets of linkers and two sets of toxins, possessing independent intellectual property rights, and have a sound patent protection strategy, including toxins, linkers, overall ADC, making them one of the strongest companies in the domestic ADC field.
It is the result of years of effort that has attracted the attention of Merck.
In May 2022, Merck acquired the Trop2 ADC drug SKB264 from Lundbeck, with a total upfront payment and milestone payments amounting to 1.363 billion dollars, as well as royalties based on the agreed net sales ratio between the two parties.
In July 2022, Merck upgraded its collaboration with Sichuan Kelun Pharmaceutical, acquiring the ADC product SKB315 targeting Claudin 18.2 with a 35 million dollar upfront payment and 0.9 billion dollar milestone payment terms.
In December 2022, Merck packaged Sichuan Kelun Pharmaceutical's early-stage ADC production line, acquiring overseas rights to 7 ADC products for an upfront payment of 0.175 billion dollars and milestone payments of 9.3 billion dollars, setting the highest record for the overseas licensing amount by a domestic innovative drug company at that time.

Behind the three ADC project collaborations with a total value of over 10 billion dollars within a year, on one hand, is Sichuan Kelun Pharmaceutical's strong ADC R&D and production technology platform, and on the other hand, is Merck's eagerness for ADC products.
During the subsequent process of Sichuan Kelun Pharmaceutical's split listing, Merck made a significant investment of 0.1 billion dollars in leading the Series B financing, obtaining a 6.95% stake in Sichuan Kelun Pharmaceutical.
02 The First Sankyo "Slanting Stab"
Although Kelun Bo Tai has been deeply tied to Merck, Merck has not placed all of its ADC resources on Kelun Bo Tai.
On October 20th last year, Merck announced an agreement with Daiichi Sankyo at a total price of 22 billion US dollars. $Daiichi Sankyo (4568.JP)$ , reaching a cooperation consensus, and gaining three new ADC drugs: patritumab deruxtecan (HER3-DXd), ifinatamab deruxtecan (B7-H3 ADC), and raludotatug deruxtecan (CDH6 ADC).
According to the agreement, Merck will pay Daiichi Sankyo 4 billion US dollars as an initial payment, and continue to pay 1.5 billion US dollars in milestone payments over the next two years. Depending on future sales targets, Merck may also pay up to an additional 16.5 billion US dollars in sales payments.
By comparing the amounts, the funds Merck invested in Daiichi Sankyo far exceed those of Kelun Bo Tai, showing that Merck values Daiichi Sankyo more.
Daiichi Sankyo is the top pharmaceutical company in Japan by market cap and has introduced three blockbuster ADC products based on its unique DXd ADC technology platform, including the 'Big Boss' DS-8201 (Enhertu), which has changed ADC industry expectations. In the middle of 2023, Enhertu's sales increased by 113% year-on-year to reach 2.315 billion US dollars, becoming the highest-selling ADC drug globally.
On the second day of the heavy collaboration between Merck and Daiichi Sankyo, Merck announced the termination of two preclinical ADC pipelines licensed from Kelun Bo Tai. Although this will not affect the upfront payment received by Kelun Bo Tai, and Kelun Pharmaceutical stated in the announcement that the returned ADC assets were not core products of Kelun Bo Tai, it still indicates a decline in Kelun Bo Tai's position in Merck's eyes.
Through cooperative layout analysis, the collaboration between Merck and Kelun Botai and Daiichi Sankyo has significant differences. Merck introduces ADC pipelines from Kelun Botai targeting mainly Trop-2, Claudin18.2, and Nectin-4, all of which are competitive targets likely to be validated in the short term; while the pipelines introduced from Daiichi Sankyo are targeting HER3, B7-H3, and CDH6, which are emerging or niche targets.
Evidently, Merck's collaboration with Daiichi Sankyo has a longer-term perspective and involves more investment, while the collaboration with Kelun Botai seems more like a one-time position, although the total amount of the collaboration is high, the down payment is not high.
On August 6th this year, Merck once again expanded its collaboration with Daiichi Sankyo to jointly develop DLL3-targeting T-cell engager MK-6070. According to the agreement, Merck will receive a $0.17 billion prepayment and certain royalties based on sales, apart from retaining rights in Japan, Merck will co-develop and commercialize globally with Daiichi Sankyo in other regions.
Coincidentally, after upgrading its collaboration with Daiichi Sankyo, Merck announced the abandonment of the Claudin18.2 target ADC pipeline SKB315 previously introduced from Kelun Botai just two weeks later, making it the third Kelun Botai ADC pipeline abandoned by Merck. Different from the two early ADC pipelines abandoned in 2023, SKB315 was one of Kelun Botai's core ADC pipelines, with high expectations from the industry, yet it was still abandoned by Merck.
Looking back over the past two years, it is not difficult to see that as Merck found Daiichi Sankyo as a new ADC partner, Kelun Botai's position in Merck's mind is gradually being marginalized.
03 Merck's harem dispute
Whether Kelun Botai or Daiichi Sankyo, Merck is pursuing maximization of its own interests.
From Seagen, Kelun Botai, to Daiichi Sankyo, Merck has repeatedly made moves in the ADC field, all in the hope of leveraging combination ADC therapy to sustain the core competitiveness of K drugs.
Relying on financial leverage, Merck has quickly established 11 ADC pipelines in research, with 6 of them already in clinical stages. The ADC-targeted therapy sector it is in has become the third largest tumor research and development sector after tumor immunotherapy represented by K meds, and precision targeted therapy represented by Olaparib and Lorlatinib.
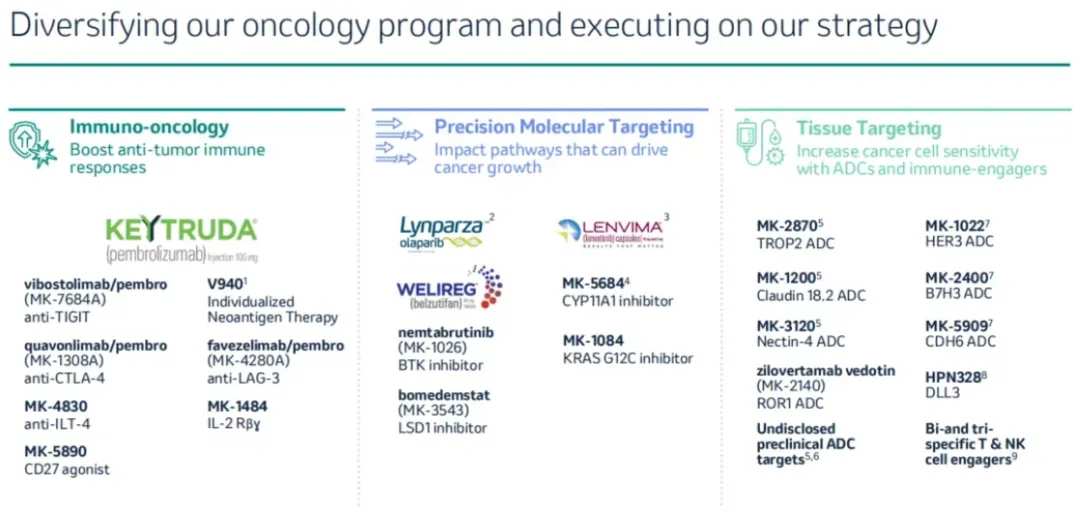
These 6 clinical ADC pipelines come from three different companies, three different technology platforms, and target 6 different points. Among them, the fastest progress is from Kelun Biotech's MK-2870 (Trop2 target point), and from Daiichi Sankyo's MK-1022 (HER3 target point), both in the state of market application.
MK-2870, as an ADC targeting TROP2, is formed by linking humanized IgG1 anti-TROP2 monoclonal antibodies and a topoisomerase I inhibitor cytotoxic drug toxin molecule KL610023. Currently, MK-2870 is in a leading position among global similar target ADCs. Merck has already launched 10 global Phase III clinical trials around it, covering multiple tumor types such as non-small cell lung cancer, endometrial cancer, breast cancer, gastric cancer, cervical cancer, etc.
The targeting of HER3 by MK-1022 is widely present in various tumor types, including breast cancer, ovarian cancer, colon cancer, gastric cancer, lung cancer, skin cancer, and pancreatic cancer. In the past, the low binding affinity of HER3 itself made drug development difficult. MK-1022 is a potential FIC product that has now been submitted for market approval, accepted by the U.S. FDA and granted priority review qualification for the treatment of locally advanced or metastatic EGFR mutant non-small cell lung cancer (NSCLC) in adults who have previously received two or more systemic therapies.
The success of K meds + Padcev has made many pharmaceutical companies realize the superior efficacy of PD-1 + ADC combination therapy over monotherapy, creating the potential of "1 + 1 > 2" efficacy. Major pharmaceutical companies are actively conducting research on PD-1 + ADC combination therapy. ADC projects have become bestsellers in the international market, with Merck being one of them. $Pfizer (PFE.US)$ Acquisition of seagen for 43 billion dollars, $AbbVie (ABBV.US)$ 10.1 billion dollarsMergerimmunogen,$AstraZeneca (AZN.US)$and$GlaxoSmithKline (GSK.US)$Please use your Futubull account to access the feature.$Johnson & Johnson (JNJ.US)$ Other pharmaceutical giants are also investing heavily in ADC projects.
From the collaboration between Merck and Daiichi Sankyo, the goal is to gain the first-mover advantage and quickly see results. The collaboration with Kelun Boai, in addition to MK-2870, seems more like a casual move: laying out several pre-clinical or early clinical projects with lower initial payments. As the projects progress, if there is commercial potential, subsequent milestone payments will continue the research and development. If they lose competitiveness, they will be promptly abandoned. The relatively low initial payment is insignificant to Merck.
For example, SKB571, selected to advance in August this year, is a new dual-antibody ADC developed to treat solid tumors such as lung cancer and gastrointestinal tumors. Although it is still in the pre-clinical stage, there are relatively few companies currently developing dual-antibody ADCs, with only dozens entering the clinical stage. In December last year, BMS acquired Bai Li Tian Heng's HER3/EGFR dual-antibody ADC for 8.4 billion dollars, setting a record for the highest initial payment for Chinese innovative drugs going global.
Daiichi Sankyo's participation has suddenly livened up Merck's ADC 'harem'. Kelun Boai, originally backed by Merck, is no longer as secure, facing the increasingly fierce competition in the heated ADC field like other biotechs. Merck, holding the 'money-printing machine' of Keytruda, can choose a more competitive ADC to replace the former product at any time.
However, Kelun Boai is not completely out of favor. MK-2870 is one of Merck's core ADC pipelines, currently pushing for multi-indication clinical trials intensively. Other early pipelines also hold Merck's BD position in fierce competition, allowing them to receive ample resources to accelerate the development progress and gain an advantage in subsequent competition.
Editor/Somer

 基于此,默沙东不断帮助K药获批新适应症,今年6月其已经获批了第40个适应症。但即使如此,K药也不是万能的,仍在一些癌种应答率不高,因此默沙东也在不断探索K药联合疗法,如与仑伐替尼联用组成的“可乐组合”,取得了有史以来肝癌治疗的最好效果。
基于此,默沙东不断帮助K药获批新适应症,今年6月其已经获批了第40个适应症。但即使如此,K药也不是万能的,仍在一些癌种应答率不高,因此默沙东也在不断探索K药联合疗法,如与仑伐替尼联用组成的“可乐组合”,取得了有史以来肝癌治疗的最好效果。