For l, if there is no shortcut, it can only rely on its own fundamentals to attract market attention. However, the core herpes zoster product has not yet been launched, and the future commercial prospects of the company are still unknown.
After the market closed on August 29, l-B (02480) officially released its 2024 interim performance. The financial report shows a net loss of 0.11 billion yuan for the period, a 38.5% increase year-on-year. Subsequently, l experienced declines in the following two trading days, with a closing decrease of 8.26% on September 2.
However, despite the fluctuations in the stock price of l, the trading volume is meager, with only 1,200 shares and 5,400 shares traded on August 30 and September 2, respectively. In fact, the total trading volume of l since the beginning of the year is only 3.4874 million shares. So far this year, except for June and July, the trading volume of l has not exceeded 0.5 million shares, and the volume in August was only 0.033 million shares. From the perspective of trading volume, l has obviously slipped to the edge of being forgotten by the market.
 In January of this year, the domestically unlisted full circulation approved by l has increased the company's market capitalization of listed in hong kong. At that time, the Sina Finance App stated, 'If calculated at the closing price of 29.70 Hong Kong dollars on January 17, the market capitalization of l's H shares reached approximately 5.99 billion Hong Kong dollars, just exceeding the inclusion market capitalization requirement of 5.75 billion Hong Kong dollars for Stock Connect. If the stock price remains above 28.5 Hong Kong dollars, it may be included in Stock Connect.'
In January of this year, the domestically unlisted full circulation approved by l has increased the company's market capitalization of listed in hong kong. At that time, the Sina Finance App stated, 'If calculated at the closing price of 29.70 Hong Kong dollars on January 17, the market capitalization of l's H shares reached approximately 5.99 billion Hong Kong dollars, just exceeding the inclusion market capitalization requirement of 5.75 billion Hong Kong dollars for Stock Connect. If the stock price remains above 28.5 Hong Kong dollars, it may be included in Stock Connect.'
Being included in Stock Connect is undoubtedly a shortcut for l to quickly increase liquidity, but it did not seize the opportunity. Since the beginning of this year, the company's stock price has been declining all the way, falling below 25 Hong Kong dollars, and the market value has also dropped to 4.583 billion Hong Kong dollars, while the threshold for inclusion in Stock Connect has reached 5.759 billion Hong Kong dollars. For l, if the stock is not sought after, Stock Connect is also out of reach.
For Lu Zhou Biotechnology, if shortcuts are not feasible, the company can only rely on its own fundamentals to attract market attention. However, the core shingles product has not yet been launched, and the future commercial prospects of the company are still uncertain.
Commercialization has reached the final stage of overcoming key obstacles.
According to the latest 2024H1 financial report released by Green Bamboo Biology, the company currently has not commercialized any products, and the current main business revenue is still 0, but the core product LZ901 has reached the critical Ph3 clinical trial stage and has recruited a total of 26,000 40-year-old and older healthy subjects in January of this year.
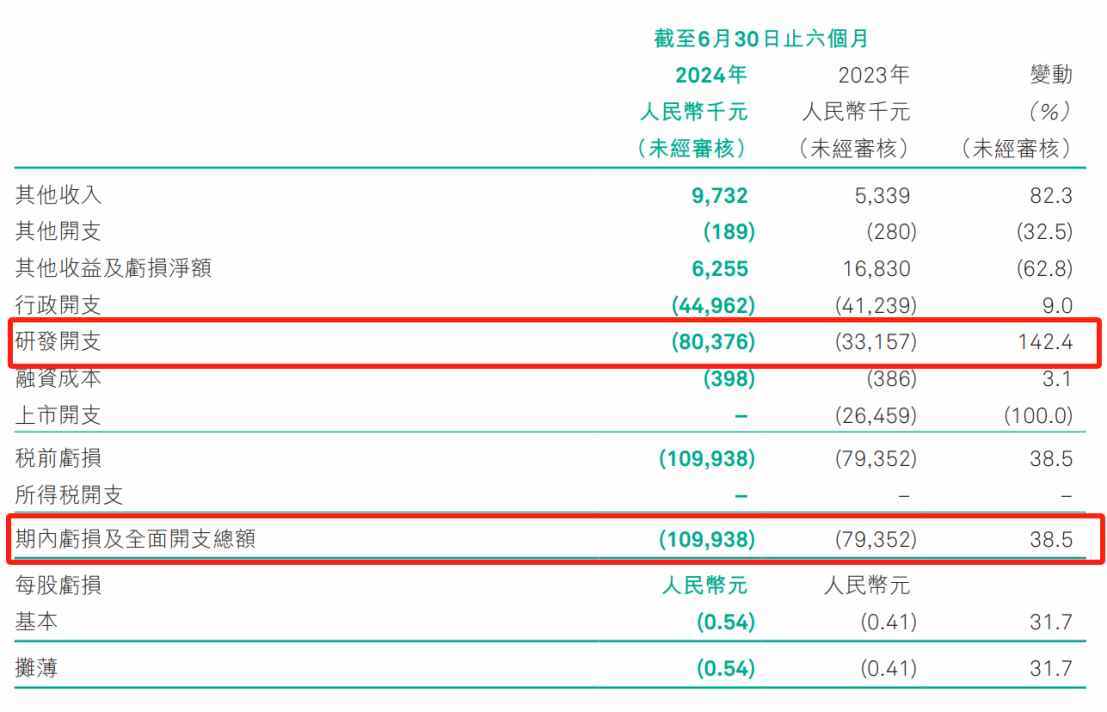
It is also for this reason that in the first half of this year, Green Bamboo Biology significantly increased its R&D expenses to 80.376 million yuan, a year-on-year increase of 142.4%, which also led to the company's current net loss reaching 0.11 billion yuan, an expansion of 38.5% year-on-year.
From the perspective of the investment logic of the Hong Kong stock market in the biomedical sector in recent years, for a non-profitable Biotech company to achieve value reassessment, the key lies in product commercialization and sustained hematopoiesis. Currently, Green Bamboo Biology is still in the first phase of promoting product commercialization.
According to the Zhixin Finance app, the recombinant herpes zoster candidate vaccine, LZ901, is currently the most core product in the research pipeline of Green Bamboo Biology. It is expected to be the world's first herpes zoster vaccine with a tetrameric molecular structure, used to prevent herpes zoster caused by varicella-zoster virus (VZV) in adults aged 50 and above.

From the pipeline progress perspective, in the short term, only this product can help the company achieve 'hematopoiesis'. The rest of the products are still in the phase I/II clinical stage, making it difficult to provide income support for Green Bamboo in the short term.
The reason why the company has high hopes for LZ901 is that it benchmarks against GSK's herpes zoster vaccine Shingrix, which continues to grow in sales and became the third largest vaccine single product in global sales last year. Currently, in the global market, due to Shingrix's stable efficacy of over 90% for all age groups in reducing infections, while Zostavax has lower efficacy and decreases with age, Shingrix quickly replaced Zostavax once it was approved for market. In 2023, sales reached as high as 3.446 billion British pounds, with a CER growth rate of 17%, and continues to maintain growth momentum.
In February last year, Lushan Biological released clinical trial data comparing LZ901 and Shringrix head-to-head, showing that LZ901 has multiple significant advantages. For example, in terms of safety, the incidence and severity of adverse reactions observed in the high/low dose groups of LZ901 were comparable to the placebo group and much lower than the Shingrix group; in terms of immune response, based on immunogenicity data collected in Phase I clinical trials of LZ901 in China, it has been proven that there is no significant difference in anti-VZV antibody levels after full vaccination with LZ901 compared to Shingrix, indicating that the immunogenicity of LZ901 is not lower than Shingrix.
From a market perspective, since Shingrix can quickly replace Zostavax with better clinical performance, LZ901 undoubtedly has the potential to accelerate the domestic substitution of Shingrix after its launch. Currently, Lushan is expected to submit a BLA application no later than January 2025 and achieve commercialization of the product in 2025 Q4.
However, looking at the current market landscape of herpes zoster in China, Lushan Biological may encounter some trouble in accelerating market expansion after the product is launched.
"Domestic popular products" have hidden worries behind the silence after one year of listing.
On the evening of August 15 this year, Bak Biological disclosed its financial report for the first half of 2024: In the first half of this year, the company achieved a revenue of 0.618 billion yuan, an increase of 10.50% year-on-year; achieved a net profit of 0.138 billion yuan, an increase of 23.54% year-on-year. If viewed by quarter, Bak Biology's revenue in the second quarter of this year decreased by 8.44% year-on-year, with a decrease in net profit attributable to the parent company by 17.16% and a decrease in non-net profit by 14.44%.
It's worth noting that in the first year of the listing of the herpes zoster vaccine, Bak Biology achieved a 70.3% year-on-year revenue increase, while the net profit attributable to the parent company and the non-net profit increased by 175.98% and 195.86%, respectively.
The current performance represents a sharp halt in the rapid growth momentum of the domestically released herpes zoster vaccine, just one year after its listing. Bak Biology's lesson from the past should not be overlooked by Lushan Biological.
In fact, Bak Biology's market performance is inferior to GSK/Chongqing Zhifei Biological's Shingrix, one reason being the clinical performance differences between the two products. Shingrix, which adopts a recombinant protein technology route, has superior protective efficacy, with a protection efficacy of 97.2% for people over 50 years old and 91.3% for those over 70 years old.
In comparison, Baik Biological's Ganwei adopts the same attenuated activity technology route as Zostavax, with the advantage of lower adverse reaction rate and wider applicability (targeting people aged 40 and above). According to Baik's own account, "the vaccine's protective efficacy is equivalent to products with the same technology route; the overall incidence of adverse reactions is lower than that of marketed products." In other words, Baik's Ganwei performs better clinically than Zostavax, but still not as good as Shingrix.
In response, Baik has adopted a "price-for-volume" market strategy. In terms of vaccination cost, Shingrix requires 2 doses, with a single dose priced at 1600 yuan, totaling 3200 yuan for the full process, while Ganwei only needs 1 dose, costing 1369 yuan, with a significant price advantage. However, the price advantage has not prevented GlaxoSmithKline's partner Zhifei from making moves in the terminal market. Data shows that with the assistance of Zhifei Biological, GSK's Xin'anlixit batch issuance increased by 117% from January to May, with 26 batches issued, compared to Baik's Ganwei which had 23 batches issued.
From the current performance of the competing products, Zhifei, relying on its advantages in terminal sales, has almost neutralized Baik's price advantage. This is undoubtedly an important experience for Green Bamboo Biotech, which has not yet launched its product.
Looking at the market landscape, in recent years, the domestic herpes zoster vaccine market is expected to be in a blue ocean, and the two domestic and imported products will not completely replace each other in the US market. However, given Zhifei Biotech's strong promotion capabilities + Shingrix's high protective efficacy, the situation where Shingrix occupies a large market share with Zhifei's assistance is unlikely to change significantly.
Although Green Bamboo Biotech's LZ901 has a relative advantage compared to Shingrix, due to the lack of first-mover advantage, it may be difficult to achieve significant volume increase in the short term through rapidly improving market share. Following that, there are multiple domestic companies including Beijing Wantai Biological Pharmacy Enterprise (603392), Walvax Biotechnology, Micucon Biotech, Cansino Biologics Inc., and Yidao Biotech, all of which are laying out plans for the herpes zoster vaccine. Even if Green Bamboo Biotech's LZ901 can successfully hit the market in Q4 next year, it remains uncertain whether it can withstand the pressure from the two existing competitors and several other products in development. Given this background, it may be quite challenging to deeply optimize Green Bamboo Biotech's financial structure and fundamentals solely relying on this product.

 今年1月,绿竹生物申请的境内未上市全流通获批,增加了公司港股流通市值。当时智通财经APP表示,“若按1月17日收盘价29.70港元计算,绿竹生物的H股市值达约59.9亿港元,刚好超过了港股通57.5亿港元的纳入市值要求,其股价只要稳定在28.5港元之上,就可能被纳入港股通。”
今年1月,绿竹生物申请的境内未上市全流通获批,增加了公司港股流通市值。当时智通财经APP表示,“若按1月17日收盘价29.70港元计算,绿竹生物的H股市值达约59.9亿港元,刚好超过了港股通57.5亿港元的纳入市值要求,其股价只要稳定在28.5港元之上,就可能被纳入港股通。”