The listing of Dapagliflozin and Metformin Hydrochloride Sustained-Release Tablets will further intensify the competition in the domestic market for antidiabetic drugs.
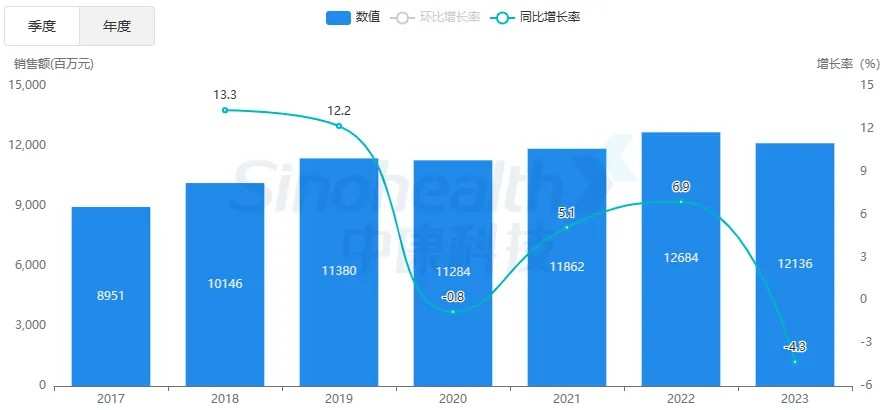
As the first approved new oral compound formulation for lowering blood sugar in China, the listing of Dapagliflozin and Metformin Hydrochloride Sustained-Release Tablets will further intensify the competition in the domestic market for antidiabetic drugs. According to the Zhongkang Kaisi system, overall, the oral antidiabetic drug retail market has remained stable, with sales of over 10 billion yuan in the past six years.
In 2023, the total sales volume of oral antidiabetic drugs in domestic tertiary hospitals and the retail market exceeds 30.8 billion yuan. In terms of the retail market, the market size of oral antidiabetic drugs is 12.1 billion yuan in 2023, with continued growth in the first half of this year and sales of nearly 6 billion yuan.
 What are the TOP 20 varieties of drugs sold in pharmacies from January to July?
What are the TOP 20 varieties of drugs sold in pharmacies from January to July?
In the period from January to July 2024, the cumulative scale of the national retail pharmacy market reached 299.1 billion yuan, a year-on-year decrease of 3.3%. Focusing on July, the scale of the national retail pharmacy market reversed its downward trend and increased by 0.7% compared to the same period last year.
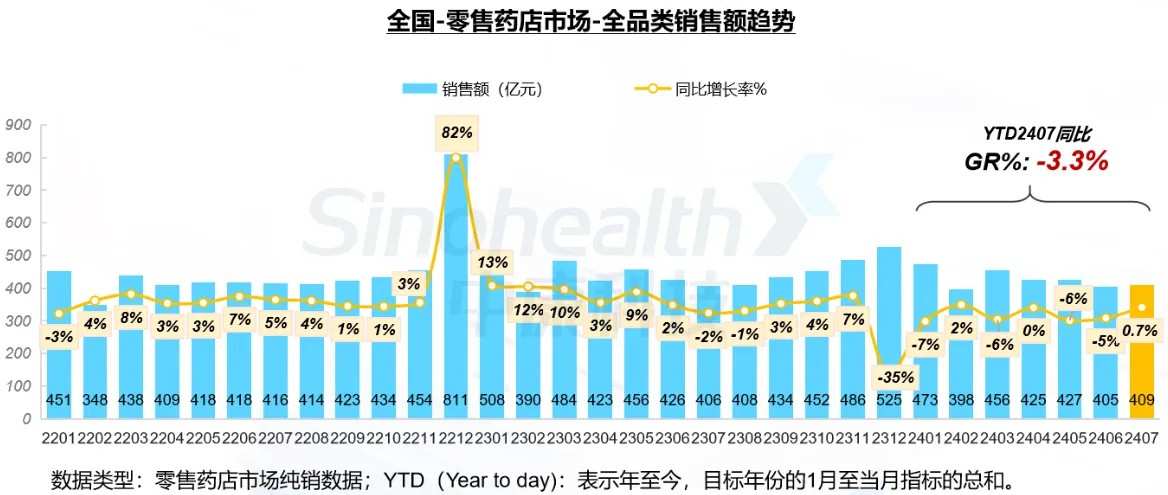
Affected by multiple factors such as economic downturn, outpatient consolidation, and the impact of e-commerce, the overall customer traffic level of retail pharmacies has been in a continuous decline since March. However, since the end of June, the rebound of the COVID-19 pandemic and the increase in related medication demand have contributed to the stabilization of customer traffic levels in July, narrowing the gap with the customer traffic levels in the same period last year.

Three batches of 693 centralized procurement varieties are officially implemented.
Recently, Anhui issued a notice that the province will start implementing three centralized procurement projects on September 30, including the Guangdong Union for scarce and emergency rescue drugs, interferon in Jiangxi, and the "3+N" Western medicine and Chinese patent medicine quantity-linked procurement in the Beijing-Tianjin-Hebei region.
Among them, the procurement cycle for the Guangdong Union is until December 31, 2026, the procurement cycle for Jiangxi Union is 4 years, and the "3+N" in the Beijing-Tianjin-Hebei region is 1 year.
If lower prices occur for the three centralized procurement projects, price linkage is required. After completing the agreed procurement quantity, the Guangdong Union prioritizes the procurement of selected products, and the usage of non-selected products should generally not exceed 30% of the total quantity of the same group of varieties. In addition to the upcoming centralized procurement projects mentioned above, Anhui will also kick off the Chinese patent medicine centralized procurement for the year 2024, which is led by the Huangshan Medical Insurance Bureau.
A total of 18 product groups and 35 varieties of Chinese patent medicine in Huangshan City have been included in the centralized procurement, including OTC varieties such as Ganmaoling capsules, Ganmaoling granules, Ganmao Qingre granules, and Qiangli Pipa lu.
With a large scale of over 1.6 billion, Sichuan Kelun Pharmaceutical became the third domestic company to win the contract.
On September 18, Sichuan Kelun Pharmaceutical announced that their generic drug, Diquinon tablets, submitted by the company, has been approved and considered to have passed the consistency evaluation. It is the third domestic generic drug company to be approved for Diquinon tablets. The database shows that the sales of Diquinon tablets in the national in-hospital market in 2023 exceeded 1.6 billion yuan.
The Levonorgestrel tablet is developed by Abbott Laboratories and is a national health insurance Class B variety. It has been approved for marketing in more than 100 countries and is used to treat related diseases caused by insufficient endogenous progesterone, such as dysmenorrhea, endometriosis, threatened abortion or habitual abortion caused by progesterone deficiency, and luteal support in assisted reproduction technology.
In terms of the layout of generic drugs, four pharmaceutical companies in Zhejiang, namely Gaizhi Medicine, Aisheng Pharmaceutical, Xianju Pharmaceutical, and Saimo Pharmaceutical, have submitted applications for four categories of generic drugs.
Hunan province rectifies the prices of unqualified products, and 67 drugs face removal from the network.
On September 18th, the Hunan Provincial Public Resource Trading Center issued a notice on price rectification for drugs listed on the network (the first batch of 2024), stipulating that drugs with minimum formulation prices higher than those of the reference formulations of the same specifications and the lowest listed prices of drugs that have passed the evaluation do not comply with the listing rules of the province and need to undergo price rectification.
Many drugs that have been named in the Hunan province's listing price unreasonable include commonly used clinical drugs, including Novartis' Valsartan Sacubitril Sodium Tablets, Baxter's Acesulfame Potassium Injection, Fosun Pharma's Fingolimod Hydrochloride Capsules, and Beihai Kangcheng's Nalatinib Malate Tablets introduced through cooperation.
Several major pharmaceutical companies question the monitoring price: lower than the centralized procurement price, and appeals are useless.
On September 18th, the Yunnan Provincial Drug Centralized Procurement Platform announced the results of the appeal and questioning of the normalization of drug listing (Batch No. 20240805): 14 companies appealed, and all the results were unsuccessful, involving well-known enterprises such as China Resources Sanjiu, Shandong Lukang Pharmaceutical, and Weicai Pharmaceutical.
The Health Bureau found that among the 14 cases, 9 cases involved appeals by enterprises regarding the rationality of applying the national monitoring price. Among them, China Resources Sanjiu questioned whether the injection of Cefuroxime Sodium for evaluation is suitable for monitoring price listing.
Some companies even directly challenge the national monitoring price. For example, Beijing Yongkang Pharmaceutical Co., Ltd. stated in the appeal that the injection solution of Nicotiamide produced by our company did not pass the audit in the injection specification hanging network, and is above the national monitoring price. Therefore, we apply to adjust the national monitoring price to 4.93 yuan per unit.
None of these appeals were successful. However, Yunnan also left a way out for the companies: from September 18th to 25th, relevant companies can consult on the results of the appeal, and those who still have objections after consultation can complain to the Yunnan Provincial Medical Security Bureau.
The latest medical equipment centralized procurement has started!
Recently, the China Government Procurement Network issued the 'Sanya Health Commission - Sanya 2024 Medical Equipment Centralized Procurement Project - Public Tender Notice'.

The document shows that the budget for the Sanya 2024 Medical Equipment Centralized Procurement Project is 55.3 million yuan. The project is divided into 4 packages, with Package A budgeting 5 million yuan, Package B budgeting 22.6 million yuan, Package C budgeting 18 million yuan, and Package D budgeting 9.7 million yuan.
This article is from 'Xinkangjie', edited by Zhitong Finance: Jiang Yuanhua.

