①Regarding the online rumors of layoffs, the secretary of the board of directors at Remegen stated that the company has been streamlining its business since last year, and optimizing positions and personnel is based on job requirements without a strict ratio. ②It was revealed that the severe myasthenia gravis indication of the targeted therapy taizhesipu will soon apply for listing. However, in the market, taizhesipu is facing multiple competitions.
"Science and Technology Innovation Board Daily" Sept 20th News (Reporter Zheng Bingxun) Recently, a rumor of layoffs that has been gradually fermenting on the internet has once again made Remegen (688331.SH) the focus of public opinion.
There are multiple versions circulating online about the layoffs at Remegen. Among them, some say the company will have two rounds of layoffs, with an expected reduction rate of 70%, while others say the number of layoffs will be nearly 1000, and some say the layoffs have already started since August this year. Of course, other layoff versions are also circulating.
 In response, the reporter of "Science and Technology Innovation Board Daily" asked Remegen's secretary, Wen Qingkai, to verify the authenticity and the ratio of layoffs. The response was, "In fact, the company has been streamlining its business since last year, and positions and personnel have been optimized based on the review situation. Optimization is only based on job requirements without a strict ratio."
In response, the reporter of "Science and Technology Innovation Board Daily" asked Remegen's secretary, Wen Qingkai, to verify the authenticity and the ratio of layoffs. The response was, "In fact, the company has been streamlining its business since last year, and positions and personnel have been optimized based on the review situation. Optimization is only based on job requirements without a strict ratio."
▌Initiate Position and Personnel Optimization
In fact, before this year, the number of employees at Remegen was still increasing, with a total of 3,332 people in 2022, increasing to 3,615 in 2023, an increase of 8.49%. However, after entering 2024, this situation began to change.
As of the first half of 2024, the number of research and development personnel at Remegen was 1,216, a decrease of 4.33% compared to the same period last year when there were 1,271 people. The proportion of R&D personnel to the total number of employees in the first half of this year was 34.77%, so the total number of employees is about 3,497, a decrease of 118 people from the end of 2023.
However, although the number of R&D personnel at Remegen decreased in the first half of this year, R&D investment is still increasing. The total R&D personnel compensation was 0.236 billion yuan, a year-on-year increase of 14.21%, the total R&D expenses were 0.806 billion yuan, a year-on-year increase of 49.18%. At the same time, sales expenses also increased by 11.28% year-on-year to 0.39 billion yuan.
Remegen indicated that due to the continuous expansion of the research product pipeline during the reporting period and the advancement of existing clinical projects, research and development expenses increased. At the same time, as the number of hospitals and covered pharmacies with access to Tai eta sipu and Videxitu monoclonal antibodies increased significantly, the expansion of first-line sales staff led to an increase in sales expenses.
Affected by this, Remegen achieved revenue of 0.742 billion yuan during the reporting period, a year-on-year increase of 75.59%. The net loss attributable to the parent company was 0.78 billion yuan, further expanding from the net loss of 0.703 billion yuan in the same period last year.
As of the first half of 2024, in addition to the approval and listing in China of four indications for Taieta sipu (RC18) for systemic lupus erythematosus, rheumatoid arthritis, and Videxitu monoclonal antibody (RC48) for HER2-expressing gastric cancer and HER2-expressing urothelial cancer, Remegen has more than 20 indications in clinical research and more than 10 pipeline projects at key/Phase III clinical stages globally.
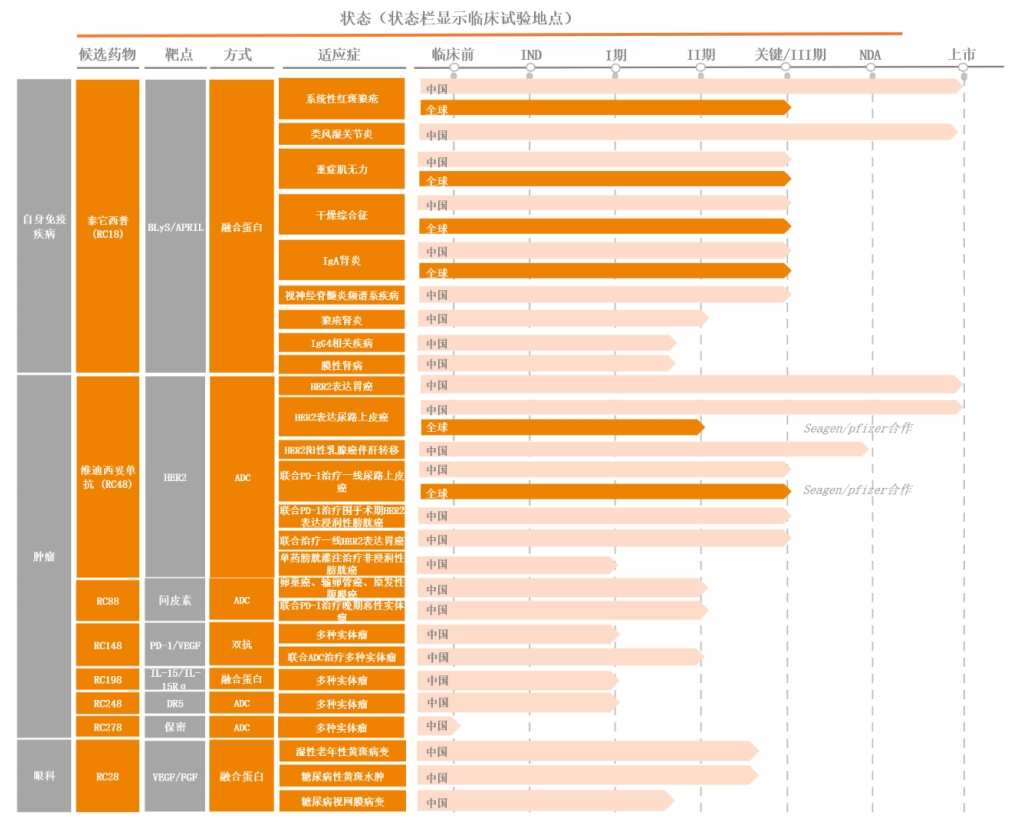
Remegen's Research Pipeline (as of the first half of 2024)
With numerous pipelines progressing simultaneously, consuming Remegen's funds, the company's year-end balance of cash and cash equivalents decreased from 2.069 billion yuan at the end of 2022 to 1.12 billion yuan in the first half of 2023, further decreasing to 0.727 billion yuan at the end of 2023, and further decreasing to 0.673 billion yuan in the first half of 2024.
In light of the growing financial pressure, Remegen disclosed a private placement plan at the end of March this year, intending to issue up to 70.7632 million shares to specific investors, raising 2.55 billion yuan to invest in 'new drug R&D projects,' specifically including 'pre-clinical research and clinical research on RC18, RC48, RC28, RC88, RC148, and RC198 products,' with a total project investment of about 2.946 billion yuan.
However, at the end of July this year, Remegen issued a revised version of the private placement plan, proposing to reduce the total fund amount to 1.953 billion yuan, with the purpose modified to be used for 'clinical research on RC18, RC48, RC28, RC88, RC148, and RC198 products,' removing the description of 'pre-clinical research' compared to the first version, and the total project investment amount was also lowered to 1.953 billion yuan.
Perhaps, it is the long-term deficit, coupled with the reduction of the private placement target, that has led Remegen to initiate optimization work on positions and personnel.
This change also seems to point to senior management. In early August of this year, Remegen announced the appointment of He Ruyi as Chief Global Strategy Officer of the company. He Ruyi will no longer serve as Chief Medical Officer and senior management, but will continue to serve as a director and core technical staff of the company.
▌gMG indications face competition from multiple sides.
For Remegen, the recent positive news may be attributed to the Phase III clinical trial of tacituzumab for the treatment of generalized myasthenia gravis (gMG), meeting the primary endpoints of the clinical trial design.
Remegen stated that there is currently no satisfactory treatment for myasthenia gravis, and effective, precise, and safe targeted biologics have become a hot spot in the research and development of myasthenia gravis drugs. Tacituzumab can simultaneously target BLyS and APRIL, directly attacking the source of pathogenic antibody production - B cells and plasma cells, thereby reducing the production of pathogenic antibodies and exerting a therapeutic effect.
Wen Qingkai revealed to the "Star Market Daily" journalist, "The company will soon apply for the market approval of tacituzumab for myasthenia gravis indications."
According to Frost & Sullivan report, the global number of myasthenia gravis patients is expected to reach 1.146 million by 2025, with approximately 0.2167 million patients in China.
According to Head Leopard Research Institute, from 2018 to 2023, the market size of myasthenia gravis drugs (MG) industry in China has increased from 1.125 billion RMB to 1.233 billion RMB. It is estimated that from 2024 to 2028, this market size will increase from 1.251 billion RMB to 1.342 billion RMB.
However, the "2022 China Myasthenia Gravis Patient Health Report" shows that for the treatment of myasthenia gravis, the predicted proportions of patients using steroids, cholinesterase inhibitors, and immunomodulators are 50%, 60%, and 31%, respectively, while the use of more expensive targeted drugs is low, expected to be only 1.3-1.5%.
Not only that, in the field of targeted drugs, Tacitusp faced many competitors.
According to the statistics of TouBao Research Institute, firstly, in 2023, AstraZeneca's Eculizumab was approved for marketing, used to treat refractory gMG adult patients with acetylcholine receptor (AChR) antibody positivity, becoming the first complement inhibitor approved for the treatment of gMG in China. Secondly, after the expiration of Roche's rituximab patent, as of June 2024, rituximab from 4 enterprises has been approved for marketing.
"Star Market Daily" reporters also found that in July of this year, Zai Lab's "Efgartigimod Alpha Injection (subcutaneous injection)" was approved by the NMPA for marketing, used for the treatment of adult gMG patients with acetylcholine receptor (AChR) antibody positivity. Before this, Efgartigimod Alpha Injection (intravenous infusion) was approved for marketing before June 2023.
In the first half of 2024, Intravenous Efgartigimod Alpha Injection achieved product revenue of $36.4 million. Zai Lab expects the product's full-year revenue to exceed $80 million.
In addition, in terms of new drug marketing applications, Tacitusp also has competitors. Among them, HengBo Pharmaceuticals' Batolimab (HBM9161) resubmitted its BLA for gMG in June 2024, and was accepted by the NMPA the following month. HengBo Pharmaceuticals predicts that Batolimab is expected to be approved in 2025.

 针对此事,《科创板日报》记者向荣昌生物董秘温庆凯求证真伪,以及裁员比例。对方回复称,
针对此事,《科创板日报》记者向荣昌生物董秘温庆凯求证真伪,以及裁员比例。对方回复称,