① Kangfang Biotech's stock price has risen 270% in the past three months. Why is the market looking for it? ② The agency drastically raised the company's target price. What do you think of the market situation in the future?
Financial Services Association, September 18 (Editor: Feng Yi) If you want to talk about popular stocks in the Hong Kong stock market recently, Kang Fang Biotech (09926.HK) is probably one that has received a lot of attention.
On September 16, Kangfang Biotech closed up 17.68%, hitting a record high of HK$72.55. Since its low during the year, Kangfang Biotech's stock price has risen by more than 270% over the past three months, and its market capitalization has skyrocketed by nearly HK$40 billion.
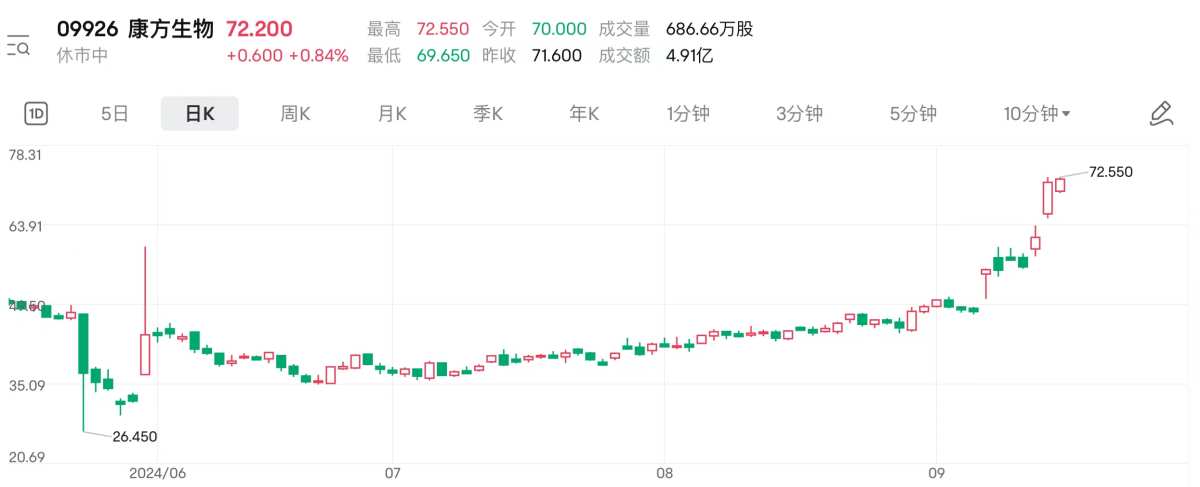
 What is more noteworthy is that Morgan Stanley recently released a report which drastically raised Health Biotech's target price from HK$64 to HK$78, once again igniting market sentiment.
What is more noteworthy is that Morgan Stanley recently released a report which drastically raised Health Biotech's target price from HK$64 to HK$78, once again igniting market sentiment.
Meanwhile, the focus of Damo's current report is on Kangfang Biotech's core pipeline variety - evosimab.
According to Damo, the Ewasi Harmoni2 data far exceeded expectations. Given Iwasi's strong data and consistency with safety, the revenue model for Kangfang Biotech was adjusted to reflect overseas revenue contributions, reducing the company's loss forecast by 30% for this year, dropping the loss forecast for next year by 17%, and raising the profit forecast for 2026 by 13%.
Earlier, Kang Fang Biotech announced the results of the Ewasi Harmoni2 study at the 2024 World Lung Cancer Congress (WCLC) on September 8.
According to data, the Evosi Harmoni2 study was the world's first randomized, double-blind, controlled phase III clinical study that compared the pharmaceutical giant MSD's ace variety pabolizumab (Keytruda, commonly known as drug K) with significant positive results.
According to the disclosed research results, the risk of disease progression/death was reduced by 49% among those who intended to be treated. Compared with pabolizumab, evosil significantly increased the objective response rate (50.0% vs. 38.5%) and disease control rate (89.9% vs. 70.5%) of first-line treatment for PD-L1 positive NSCLC patients, showing its efficient anti-tumor effects.
Simply put, Evosi showed almost twice the efficacy of K drugs in this experiment.
You need to know that as the best-selling anti-cancer drug in the world, K Pharmaceuticals will reach 25 billion US dollars in global sales in 2023, and is dubbed the “World Drug King”. According to MSD's financial report, in the first half of 2024, K Pharmaceuticals's global revenue reached 14.217 billion US dollars, an increase of 18% over the previous year.
As a competitor of K Pharmaceuticals, Evarosi naturally made investors very excited in the face of huge market prospects. This is not difficult to explain why the company's stock price has soared recently.
According to research by Huafu Securities analyst Chen Tielin, after Evosi's overseas phase 3 clinical certainty was highly improved, risk-adjusted Eversi's US lung cancer sales peaked at more than 4.5 billion US dollars.
Furthermore, Huafu Securities believes that the field of lung cancer is only the beginning of global clinical development of Evosi, and its overseas value still needs to be reassessed. The company's revenue for 2024-2026 is estimated to be $2.65, 4.06 and 6.42 billion, respectively, and the estimated reasonable share price is HK$81.89.
However, although investors' expectations for Kangfang Biotech and the drug Eversi have continued to be hot in the near future. However, after the stock price reached a new high, there were also quite a few questioning voices in the market.
On the one hand, as early as December 2022, Kangfang Biotech granted agent Summit Therapeutics an exclusive license to develop and commercialize evosimab in the US, Canada, Europe and Japan with a down payment of 0.5 billion US dollars, a total transaction amount of up to 5 billion US dollars, and a low double-digit commission on net sales. This deal also once set a new record for overseas business expansion for innovative domestic pharmaceutical companies.
In June 2024, the two sides also signed a supplementary license agreement to expand the scope of the licensing market for Evosi under the original cooperation agreement. Summit added exclusive rights for the development and commercialization of Iwasi in related markets such as Central America, South America, the Middle East and Africa. Kangfang Biotech received a down payment of 70 million dollars and a milestone payment, as well as a sales commission from Evosi in the newly licensed market.
In other words, in fact, Kangfang Biotech has already realized most of Ewasi's overseas commercialization potential through commercial expansion.
According to MSD's financial report, its total global revenue for the first half of 2024 was 31.887 billion US dollars, of which K Pharmaceuticals accounted for about 44.6% of revenue. In the same period, MSD's total revenue in China was US$3.534 billion, accounting for 12.4% of global revenue.
This shows that the main demand for K-drug is that it sells well from overseas markets. Assuming that, as expected by the market, Iwasi can catch up with K drugs in terms of commercialization in the future, how much profit Kangfang Biotech can obtain when it has already granted most of its overseas rights also needs further evaluation.
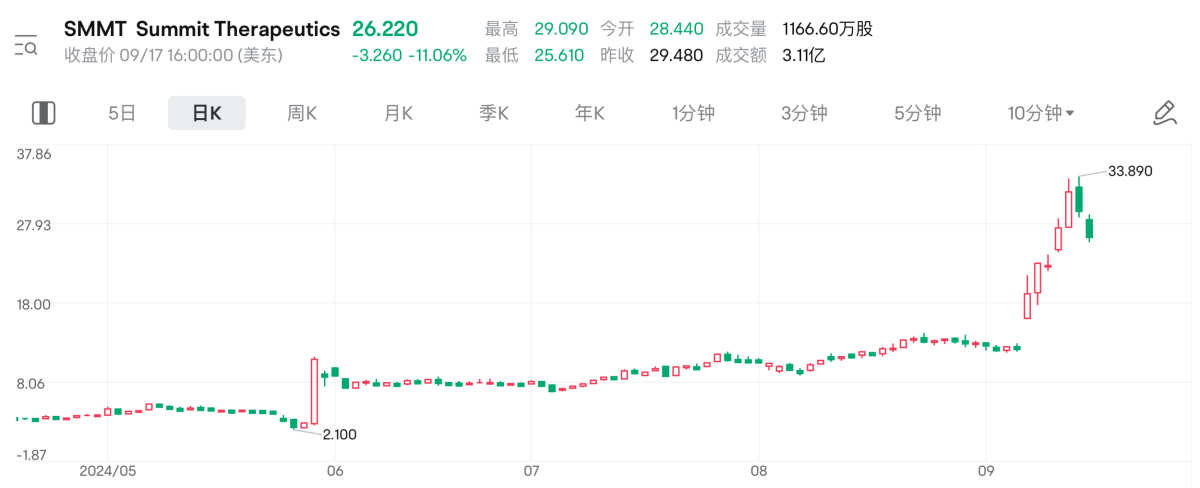
In fact, since Iwasi received attention from inside and outside the industry, the stock price of its overseas agent Summit has skyrocketed about 16 times from its lowest point. The total market value has exceeded 19 billion US dollars, which is far higher than the market value of Kang Fang Biotech's Hong Kong stock. This also validates the investment logic that Evosil is comparable to K-drugs, and that the potential benefits come more from overseas markets.
On the other hand, MSD K was launched in 2014. Over the past ten years, this drug has been approved for 40 indications worldwide, which is the key to its annual sales reaching tens of billions of dollars.
Domestically, K-drug has also reached 14 approved indications in China, covering the treatment of melanoma, lung cancer, esophageal cancer, colorectal cancer, head and neck cancer, stomach cancer, liver cancer, breast cancer, biliary tract cancer, and MSI-H solid tumors.
In contrast, Evosi has currently been approved for 1 indication in China, and 8 phase III clinical studies are being carried out. Of these, 2 international multi-center phase III clinical trials are being carried out overseas, and 6 are phase III clinical studies using PD- (L) 1 monoclonal antibody as a positive control drug.
Furthermore, as the commercialization process of Evosi deepens, it is also worth paying attention to whether K Pharmaceuticals, as an established product, will meet the challenges through price strategy adjustments.
Overall, it can be said that Kangfang Biotech's recent strong market is almost entirely dependent on the market's commercial expectations for Iwasi. This not only proves the progress of China's innovative drug industry at the technical level, but the extent to which the prospects of domestic innovative drug companies depend on overseas markets also requires careful evaluation by investors.

