- For printing use.
- September 12, 2024.
National Cancer Center, a national research and development organization
Eisai Co., Ltd.
Key Points of Announcement:
- We conducted a cross-cancer evaluation of E7820, Eisai's targeted protein degradation inducer, using patient-derived tumor tissue transplantation (PDX) models (pancreatic cancer, bile duct cancer, stomach cancer, uterine corpus cancer) transplanted into immunodeficient mice, and observed tumor shrinkage rates of 38.1% overall, 58.3% for bile duct cancer, and 55.6% for uterine corpus cancer.
- PDX models have a high predictive accuracy for treatment effectiveness and show a high similarity between clinical data and PDX model responsiveness. Therefore, this research result suggests the tumor shrinkage effect of E7820 in bile duct cancer and uterine corpus cancer.
- We performed whole exome sequencing of the PDX model and identified genetic mutations that can be potential biomarkers for predicting the efficacy of E7820.
- This research achievement has been published in the peer-reviewed academic journal 'npj Precision Oncology'.
- Based on the information of the cancer types and biomarkers for which the effectiveness of E7820 identified in this study is expected, physician-led clinical trials will be initiated at the National Cancer Center Hospital Chuo and Higashi Hospitals to evaluate safety including tolerability and exploratory effectiveness in Japanese patients.
- By using the J-PDX (Japanese patient-derived xenograft) library of the National Cancer Center for drug discovery and development, it has become possible to quickly identify efficacy evaluation and predictive biomarkers for each cancer type, and even launch clinical trials in an integrated and rapid manner. Going forward, we aim to establish a drug discovery research system that will accelerate the development of novel anticancer drugs by accumulating such cases.
 Overview
Overview
The National Cancer Center (located in Chuo-ku, Tokyo; Director: Hitoshi Nakagama) and Eisai Co., Ltd. (headquarters: Bunkyo-ku, Tokyo; Representative Corporate Officer and CEO: Haruo Naito) are conducting research on the establishment of a drug discovery research system that will accelerate the development of novel anticancer drugs, with support from the Practical Research for Innovative Cancer Control program (CiCLE) of the Japan Agency for Medical Research and Development (AMED) since 2021.
In this study, the National Cancer Center and Eisai conducted non-clinical trials on the novel drug candidate E7820 created by Eisai, using the J-PDX library of tumor tissues derived from Japanese cancer patients engrafted into immunodeficient mice. The efficacy of E7820 was evaluated in 42 PDX models (12 pancreatic cancer models, 12 biliary tract cancer models, 9 gastric cancer models, and 9 uterine corpus cancer models).
As a result, tumor shrinkage was observed in 16 out of 42 models (38.1%) with E7820 at a dose of 100 mg/kg, 7 out of 12 biliary tract cancer models (58.3%), and 5 out of 9 uterine corpus cancer models (55.6%). PDX models have high predictive accuracy for therapeutic effects and show a high degree of similarity between clinical data and PDX model responsiveness, suggesting the tumor shrinkage effect of E7820 in biliary tract cancer and uterine corpus cancer in this study.
Furthermore, in order to explore biomarkers correlated with the tumor shrinkage effect of E7820, whole exome sequencing was performed on the PDX models. As a result, frequent mutations in homologous recombination repair (HRR)-related genes, such as BRCA1, BRCA2, or ATM, which are one of DNA repair mechanisms, were observed in the PDX models that showed tumor shrinkage effect. This suggests that these gene mutations have the potential to be biomarkers for the effectiveness of E7820.
This research achievement has been published in the peer-reviewed academic journal 'npj Precision Oncology'.
Based on these results, the National Cancer Center Central Hospital (Director: Yasuyuki Seto) and Toho Hospital (Director: Toshihiko Doi) have initiated the Phase I physician-led trial (NCCH2303) to evaluate the safety and efficacy of E7820 in Japanese patients with solid tumors. After confirming the tolerability of E7820 and determining the recommended usage and dosage, the National Cancer Center and Eisai will consider conducting Phase II trials to confirm the effectiveness of E7820 in solid tumors with specific cancer types or biomarkers, and further explore the possibility of applying for regulatory approval. In addition, we aim to establish the system built in this study as a drug discovery research system to accelerate the development of novel anticancer drugs.
Background
The PDX model is one of the cancer models created by directly transplanting tumor tissue from cancer patients into immunodeficient mice. It is widely used in non-clinical trials and research. The PDX model maintains much of the heterogeneity and genetic mutations of the original tumor, and has been reported to have a higher predictive accuracy of treatment efficacy compared to traditional cell lines or models created by transplanting cell lines into mice. There is a high degree of similarity between the clinical data and the responsiveness of the PDX model.
The National Cancer Center Research Institute has established the J-PDX library derived from Japanese cancer patients with accompanying clinical information in 2020, and currently holds 651 PDX models across various cancer types (as of July 3, 2024). These models are being actively used in drug discovery and development.
Eisai considers the field of oncology as one of its strategically important areas, leveraging its experience with approved microtubule dynamics inhibitor eribulin mesylate (brand name: Halaven) and multikinase inhibitor lenvatinib mesylate (brand name: Lenvima) to create innovative new drugs with new targets and mechanisms of action under the Deep Human Biology Learning drug discovery system, contributing to the realization of cancer cures.
E7820 acts as a molecular glue that selectively binds to DCAF15, which is involved in selective protein degradation, and the splicing factor RBM39. It is a sulfonamide-based anticancer agent that selectively degrades RBM39. This action induces abnormalities in RNA splicing and is expected to inhibit cancer cell proliferation. Clinical trials using E7820 have been conducted overseas.
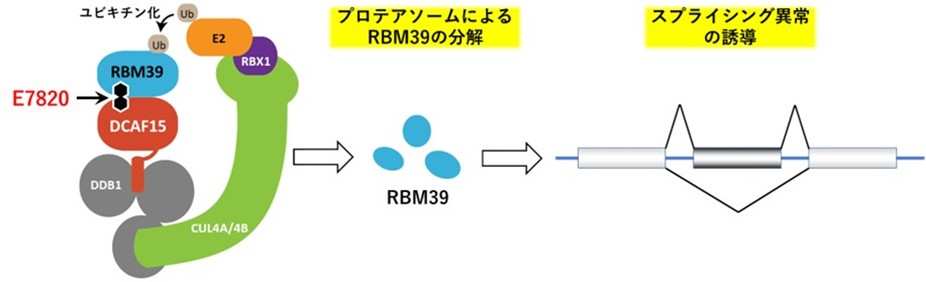
Figure 1 E7820 binds splicing factor RBM39 to the ubiquitin ligase DCAF15. RBM39 incorporated into the ubiquitin ligase complex is ubiquitinated and degraded by the proteasome. The degradation of RBM39 induces splicing abnormalities, leading to an antitumor effect.
National Cancer Center and Eisai are conducting non-clinical trials using J-PDX across organs, as well as physician-led clinical trials for rare and refractory cancers, aiming to confirm their clinical utility and seek approval for Eisai's new drug candidates in the research on drug discovery methods to accelerate the development of anticancer drugs for rare and refractory cancers (Figure 2). Furthermore, they are establishing PDX from tumor tissues before and after treatment, conducting comparative analysis of drug responsiveness and cancer genomes, and working on exploring novel drug targets and elucidating mechanisms of drug resistance to advance into new drug discovery. Through these efforts, they aim to establish a drug discovery system to accelerate the development of novel anticancer drugs.
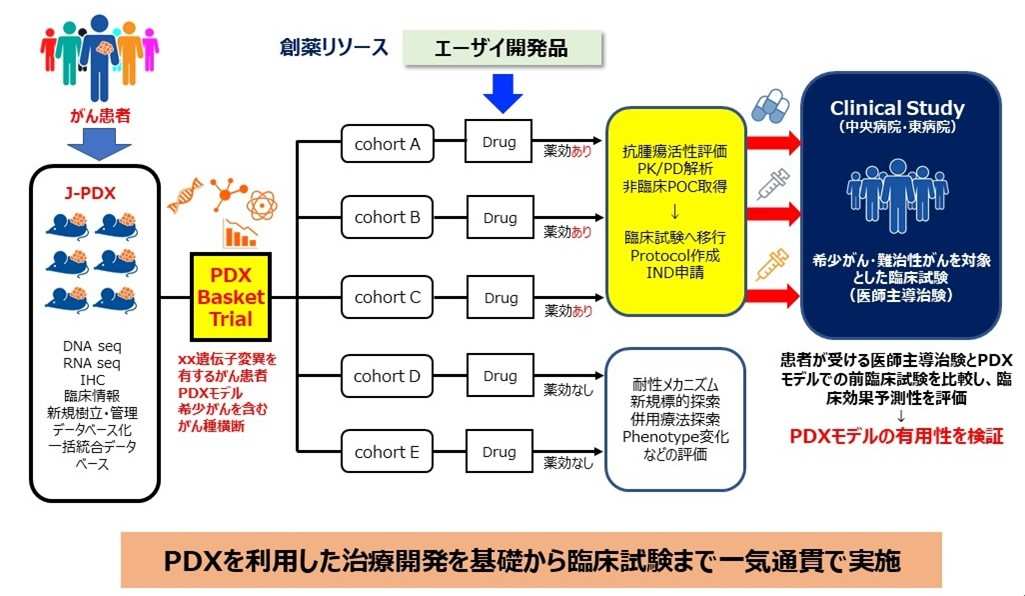
Figure 2 Overview of research and development between National Cancer Center and Eisai
May 14, 2021 Press Release
National Cancer Center and Eisai Co., Ltd. Start Research on Drug Discovery Methods to Accelerate the Development of Anticancer Drugs for Rare and Refractory Cancers using PDX and Cancer Genome Data with High Predictive Power of Treatment Effects
Research Results
Research Overview
Evaluation screening of E7820 using PDX mouse models.
E7820 was orally administered at doses of 100 mg/kg or 200 mg/kg for 21 consecutive days to a total of 42 PDX models (12 pancreatic cancer models, 12 biliary tract cancer models, 9 gastric cancer models, 9 uterine corpus cancer models), and screening evaluations were conducted.
As a result, significant tumor shrinkage (tumor volume growth rate < -30% in the group treated with the drug) was observed (Figure 3a). The overall response rate was 38.1% (16 cases) with 100 mg/kg dose and 54.8% (23 cases) with 200 mg/kg dose. The highest response rate at 100 mg/kg dose was observed in biliary tract cancer (58.3%), followed by uterine corpus cancer (55.6%) and gastric cancer (33.3%). The lowest response rate was observed in pancreatic cancer at 8.3%, indicating that the effect varies depending on the type of cancer (Figure 3b).



By screening the effectiveness of E7820 using a PDX mouse model and conducting whole-exon analysis, the types of cancers and biomarkers indicating the sensitivity of E7820 to the drug were identified (Figure 3).
HRR-related gene abnormalities are frequently observed in PDX models sensitive to E7820.
To identify molecular markers associated with the sensitivity of E7820, whole-exon sequencing and whole-transcriptome analysis were performed (Figure 3a). Mutation analysis using whole-exon sequencing revealed a high frequency of mutations in HRR-related genes such as BRCA1, BRCA2, and ATM in PDX models with observed drug effects. Comparing the sensitivity of PDX models with mutations and those without mutations, ATM mutations were significantly enriched in the sensitivity group (p=4.5×10-3, FDR=0.14), and BRCA2 mutations also showed a tendency to be enriched in the sensitivity group (p=4.8×10-2, FDR=0.51) (Figure 3c). In contrast, TP53 mutations were significantly observed in the non-sensitivity group (p=5.9×10-3, FDR=0.14), and this enrichment was suggested to be related to the high mutation positivity rate of TP53 in pancreatic cancer.
Long-term administration of E7820 inhibits the proliferation of homologous recombination deficiency (HRD) positive cells.
Recent reports have shown that E7820 affects the RNA splicing of several HRR genes, but it has not been shown that HRD, which is the loss of function of the HRR pathway, is a biomarker predicting the responsiveness of E7820. In the screening using PDX models, significant tumor shrinkage was observed in HRD-positive tumors, suggesting that long-term exposure to this compound may result in growth inhibition. Therefore, the efficacy of E7820 was evaluated by short-term culture (3 days) or long-term culture (12 days) of colon cancer cell lines with or without BRCA2 knockout (DLD1-BRCA2-KO and DLD1-P, respectively) (Figure 4a). DLD1-BRCA2-KO cells showed higher sensitivity to E7820 on day 12 compared to DLD1-P cells.
To further investigate whether other genes involved in DNA damage response affect the sensitivity to E7820, ATM, ATR, or BAP1 genes were knocked out in DLD1-P cells and then treated with the drug. It was revealed that DLD1-P cells became sensitive to E7820 (Figure 4b).

BRCA2 dysfunction increases DNA double-strand breaks by E7820.
Due to the sensitivity of DLD1-BRCA2-KO to E7820, exposure to E7820 was considered to cause DNA damage. The expression of γH2AX, a marker of DNA double-strand breaks, was evaluated in cells cultured for 72 hours with E7820 (1μM) or the PARP inhibitor olaparib (1μM), showing that E7820 administration induced more γH2AX appearance than olaparib administration (Figure 4c).
Transcriptome changes associated with E7820 administration
To clarify how E7820 induces DNA double-strand breaks, a whole transcriptome analysis was performed to evaluate genes that were commonly upregulated or downregulated by E7820 treatment in six cancer cell lines. As a result, 1,655 upregulated genes (fold change >1.1) and 2,787 downregulated genes (fold change <0.9) were observed in three or more cell lines. The internally enriched cell pathways of the downregulated genes included DNA damage repair-related signaling pathways such as "nucleotide excision repair," "hereditary breast cancer-related signals," and "BRCA1-associated DNA damage response" (Figure 5a). These pathways contained genes related to HRR such as PALB2, BRIP1, BRCA1, RAD50, MRE11, ATR, FANC family genes, as well as genes essential for nucleotide excision repair (NER) such as XPC and ERCC2. Subsequently, examination of splicing abnormalities induced by E7820 showed that the most increased splicing abnormality in both DLD1-P and DLD1-BRCA2-KO cells was intron retention following drug administration, while the most decreased splicing abnormality was single exon skipping (Figure 5b). Among the 41 downregulated genes related to DNA repair, 25 genes (61%) showed induced intron retention by drug treatment (Figure 5c).

Analysis of transcriptome changes associated with E7820 administration revealed decreased expression of genes related to DNA damage repair signaling pathways and induction of intron retention.
Mis-splicing of mRNA has a significant impact on the translation of the corresponding mature proteins. Therefore, the protein levels of FANCD2 and FANCA in DLD1-BRCA2-KO cells were measured (Figure 5d). Upon E7820 administration, the levels of FANCD2 and FANCA proteins decreased significantly in a time-dependent manner. Importantly, the reduction in FANCD2/FANCA protein levels coincided with the accumulation of γH2AX and caspase-3 cleavage (a hallmark of cell apoptosis).
About the use of drug discovery using PDX (patient-derived xenograft) models.
One of the challenges in previous anticancer drug development is the low predictive ability of experimental models using cell lines for therapy effect prediction. On the other hand, PDX is an animal model that reproduces tumors by transplanting cancer tissue from cancer patients into immunodeficient mice, and it has been reported to have a high reproducibility rate because it can retain the characteristics of cancer tissue, leading to rapid progress in drug discovery. The National Cancer Center has confirmed that the effectiveness of anti-HER2 therapy in PDX models created from uterine cancer sarcoma patients matches with the clinical efficacy.
The large-scale J-PDX library, derived from Japanese cancer patients and constructed by the National Cancer Center, has the following features: 1. Establishment of PDX across various organs, focusing not only on common cancers but also on rare cancers such as osteosarcoma and rhabdomyosarcoma, as well as cancers prevalent in Asia. 2. Establishment of PDX not only from surgical specimens but also from specimens during drug resistance periods. 3. PDX accompanied by detailed clinical information, including treatment history. It is expected to contribute to the promotion and acceleration of new anticancer drug development.
Press release on April 10, 2023.
Confirmation of the effectiveness of anti-HER2 therapy with trastuzumab deruxtecan in uterine cancer sarcoma, consistent with the predictive effect in PDX models, opens the path to the development of treatment for rare cancers.
Publication paper
Journal name: npj Precision Oncology
Title: A molecular glue RBM39-degrader induces synthetic lethality in cancer cells with homologous recombination repair deficiency
Authors: Shinji Kohsaka, Shigehiro Yagishita, Yukina Shirai, Yusuke Matsuno, Toshihide Ueno, Shinya Kojima, Hiroshi Ikeuchi, Masachika Ikegami, Rina Kitada, Ken-ichi Yoshioka, Kohta Toshimitsu, Kimiyo Tabata, Akira Yokoi, Toshihiko Doi, Noboru Yamamoto, Takashi Owa, Akinobu Hamada, Hiroyuki Mano
| National Cancer Center | |||
| Institute | Cell Informatics Field | Field Director | Shinji Kohsaka |
| Institute | Cell Informatics Field | Unit Leader | Ueno Toshihide |
| Institute | Cell Informatics Field | Chief Researcher | Ikegami Masachika |
| Institute | Cell Informatics Field | Voluntary trainee | Yukina Shirai |
| Institute | Cell Informatics Field | Voluntary trainee | Susumu Kojima |
| Institute | Cell Informatics Field | Voluntary trainee | Rina Kita |
| Institute | Field of molecular pharmacology research | Field Director | Tetsu Hisanada |
| Institute | Field of molecular pharmacology research | Unit Leader | Kaori Yanagimoto |
| Institute | Genome Stability Control Research Unit | Unit Leader | Kenichi Yoshioka |
| Institute | Genome Stability Control Research Unit | JSPS research fellow | Yusuke Matsuno |
| Institute | Genome Stability Control Research Unit | Visiting researcher | Rika Matsuo |
| Institute | Director | Hiroshi Kanono | |
| Central Hospital | Advanced Medical Department | Chief | Noboru Yamamoto |
| East Hospital | Advanced Medical Department | Chief | Toshihiko Doi |
| Eisai Co., Ltd. | ||
| DHBL Cell Lineage & Differentiation domain | Researcher | Kota Kikumitsu |
| DHBL Cell Lineage & Differentiation domain | Researcher | Kimoyo Tabata |
| DHBL Cell Lineage & Differentiation domain | Head | Akira Yokoi |
| Chief Scientific Officer | Executive Officer | Takashi Yamato |
DOI: 10.1038/s41698-024-00610-0
Publication Date: May 24, 2024
URL: (Links to an external site)
About the Phase I clinical trial in Japan, led by domestic doctors, to evaluate the safety and efficacy of E7820 for solid cancers in Japanese individuals.
In this trial, in the dose confirmation part, the tolerance, pharmacokinetics, and other characteristics of E7820 will be confirmed in Japanese individuals with solid cancers who are unresponsive or intolerant to standard treatment. In the expansion part, the efficacy and safety of E7820 will be exploratively evaluated using the dosing regimen that has been confirmed for tolerance in the dose confirmation part in patients with biliary tract cancer, uterine corpus cancer, and solid cancers with homologous recombinant repair gene mutations. The trial will be conducted with the provision of the investigational drug from Eisai. In overseas settings, clinical trials using E7820 have been conducted, and the maximum tolerated dose (MTD) with once-daily oral administration is considered to be 100 mg.
Study Name
Phase I clinical trial led by domestic doctors to evaluate the safety and efficacy of E7820 for solid cancers in Japanese individuals.
(Clinical trial implementation plan number: NCCH2303, trial abbreviation: CIRCUS)
Investigational Drug Used
E7820 (Oral medicine: sulfonamide-based splicing control agent)
The physical condition of the patients who can participate in the clinical trial (patient selection criteria)
1. Written consent is obtained
2. Diagnosed with unresectable advanced or recurrent solid cancer
3. Diagnosed with no standard treatment, or unresponsive or intolerant to standard treatment
4. At the time of registration for the clinical trial, the age is 18 years or older
5. Each organ function is maintained within the specified range
Note: The above patient selection criteria are summary and even if they meet the above, they may not be able to participate in this clinical trial, please understand.
Principal Investigator
Noboru Yamamoto (Director, Advanced Medical Department, National Cancer Center Hospital)
List of participating facilities in physician-led trial (CIRCUS trial)
National Cancer Center Hospital Central Hospital (5-1-1 Tsukiji, Chuo-ku, Tokyo)
National Cancer Center Hospital East Hospital (6-5-1 Kashiwanoha, Kashiwa City, Chiba)
Clinical research implementation plan and research overview publication system
jRCT number: jRCT2031240210
For detailed information on this trial, please verify from the clinical research implementation plan and research overview publication system.
(Link to an external site)
Research expenses
This trial is supported by the following businesses.
Japan Agency for Medical Research and Development (AMED) Medical Research and Development Innovation Base Creation Project (CiCLE) (JP20pc0101051)
Based on the results of this demonstration, Hitachi High-Tech and Hitachi will collaborate with various stakeholders such as material manufacturers including Sekisui Chemical to develop a more user-friendly environment and challenge One Hitachi to commercialize services using this system in Fiscal Year 2025. After the service is commercialized, Hitachi High-Tech will use its broad distribution network as a sales agent to expand to domestic and overseas product and material manufacturers. Companies (buyers and sellers) that participate in this business are being asked to join. Hitachi will work with Hitachi High-Tech and Sekisui Chemical to promote system development toward commercialization. Sekisui Chemical will continue to actively promote initiatives toward achieving a circular economy in 2050 by collaborating with Hitachi High-Tech and Hitachi.
By using the J-PDX library of the National Cancer Center in drug discovery and development at the company, it has become possible to quickly realize the evaluation of drug efficacy and the identification of predictive biomarkers for novel drug candidates, and even the launch of clinical trials. In this trial, after confirming the safety including the tolerability of E7820 and determining the recommended dose, the National Cancer Center and Eisai will consider conducting Phase II trials to confirm the effectiveness against solid cancers with specific cancer types or biomarkers, and even preparing for application for approval, aiming for drug approval.
In addition, we aim to establish the system built in this study as a drug discovery research system that accelerates the development of new anticancer drugs.
Glossary
Note 1: PDX (patient-derived xenograft): Mouse model
It is one of the cancer models created by directly transplanting tumor tissue from cancer patients into immunodeficient mice.
Note 2: Biomarkers
Molecules such as proteins and genes that serve as indicators of the presence of a disease or the selection of a treatment method.
Note 3: Whole Exome Sequencing
A method for selectively enriching the exonic regions that encode proteins within the human genome and analyzing them efficiently.
Note 4: Homologous Recombination Repair
One of the main mechanisms in DNA double-strand break repair, which uses the sister chromatid as a template for repair.
Note 5: Physician-Led Investigational Trial
In order for a new drug to be approved and covered by insurance, clinical development (clinical trials) of the new drug is necessary. In the case of pharmaceutical products that are promising but not undergoing clinical trials under corporate leadership, physician-led trials are conducted with the cooperation of pharmaceutical companies, where the physicians themselves conduct the trials.
Note 6: Tolerability
Tolerability refers to the degree to which adverse reactions caused by the investigational drug are considered acceptable in humans. Specifically, the frequency and severity of adverse reactions that are considered difficult to tolerate when the investigational drug is administered to humans are taken into consideration.
Note 7: RNA Splicing
RNA splicing is an essential mechanism for gene expression that removes introns from pre-mRNA to produce mature mRNA.
Note 8: Whole Transcriptome Analysis
Whole transcriptome analysis is a method for analyzing the sequence and expression levels of all gene transcripts (transcriptome) present in a cell.
Note 9: PARP inhibitor
By inhibiting poly adenosine 5'-diphosphate ribose polymerase (PARP), an enzyme that plays an important role in DNA repair and other cellular functions, synthetic lethality is induced in tumor cells with mutations in homologous recombination repair genes.
Contact
Inquiry regarding non-clinical trials
National Cancer Center, National Research and Development Corporation.
Research Institute, Cell Informatics Department
Shinji Kosaka
Phone number: 03-3542-2511 (Switchboard)
E-mail: skohsaka@ncc.go.jp
Inquiries regarding physician-led clinical trials
National Cancer Center, National Research and Development Corporation.
Clinical Research Support Department, Research Planning and Promotion Department, International Research Support Office, Central Hospital
Telephone number: 03-3547-5201 (extension 2686)
Email: ncch2303_office@ml.res.ncc.go.jp
Public Relations Contact
National Cancer Center, National Research and Development Corporation.
Planning Strategy Bureau, Public Relations Planning Office
Phone number: 03-3542-2511 (Switchboard)
Email: ncc-admin@ncc.go.jp
Eisai Co., Ltd.
PR Department
Phone number: 03-3817-5120
- Full release

