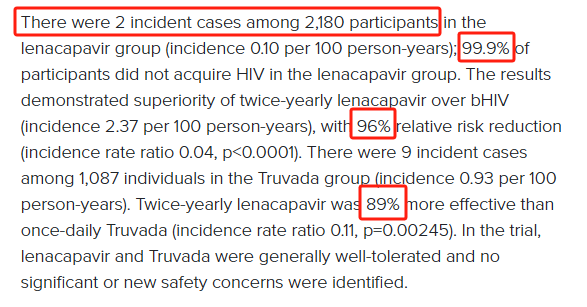
① The results showed that out of 2,180 subjects in the lenacapavir group, there were only 2 new cases, and 99.9% of participants were not infected with HIV, which reduced the relative risk by 96%; ② The press release also mentioned the “PURPOSE 1” trial results announced in June — among the 2134 women in the lenacapavir group, there were “zero cases” of HIV infection.
Financial Services Association, September 13 (Editor: Zhao Hao) On Thursday (September 12) local time, Gilead Sciences, Inc. (Gilead Sciences, Inc.) of the United States announced on its official website that its new drug lenacapavir (lenacapavir) has once again obtained amazing experimental results, showing extremely high efficacy in preventing HIV (HIV).
The latest published results are a phase 3 double-blind, multicenter, randomized study trial called “PURPOSE 2,” which aims to evaluate the safety and efficacy of Lenacapavir and the old drug Truvada (emtricitabine tenofovir tablets) for HIV pre-exposure prophylaxis (PrEP).

Source: Gilead Sciences official website
According to the press release, more than 3,200 men aged 16 or above were treated with LenaCapavir and Truvada at a ratio of 2:1. There are 88 test sites in Argentina, Brazil, Mexico, Peru, South Africa, Thailand and the United States.
The results showed that out of 2,180 subjects in the lenacapavir group, there were only 2 new cases, and 99.9% of participants were not infected with HIV, which reduced the relative risk by 96%; as a control, there were 9 new cases among the 1,087 subjects in the Truvada group.
Source: Gilead Sciences official website
Gilead said that in the study, LenaCapavir was 89% more effective than Truvada. According to information, Truvada is an oral medication taken once a day; lenacapavir is a capsid inhibitor that is injected subcutaneously every six months.
The press release also mentioned the “PURPOSE 1” test results announced in June. Unlike “PURPOSE 2,” this experiment investigated the effectiveness of lenacapavir on women. The data showed that among the 2,134 women in the lenacapavir group, there were “zero cases” of HIV infection.

It is worth mentioning that due to lenacapavir's excellent performance, in “PURPOSE 1” and “PURPOSE 2,” the independent data monitoring committee recommended that Gilead end the double-blind phase of the trial early and provide open label treatment with the drug to all participants.
Gilead Chairman and CEO Daniel O'Day wrote in a press release, “Both Phase III studies have achieved such remarkable results, and lenacapavir has proven its potential to change the way HIV is prevented and help end AIDS.
“Now that we have a comprehensive data set covering multiple study groups, Gilead will urgently work with regulators, governments, public health, and community partners to ensure that, as long as approval is obtained, medicines can be provided to everyone who wants or needs them around the world.”
Onyema Ogbuagu, the main researcher at PURPOSE 2, said that taking oral medication every day can cause difficulties such as missed doses and discrimination, which weakens the effectiveness of pre-exposure prophylaxis. Lenacapavir's breakthrough “greatly enhances our toolkit and brings us closer to achieving an AIDS-free generation.”
Michael Yee, an analyst at the US investment bank Jefferies, commented that lenacapavir's data showed consistent and steady results in the two studies and different groups. The test results should lead to FDA approval of the drug. Yee expects it to be marketed in 2025.
