Insolvent Yingen Biotech currently has only one path to follow: seek listing on the Hong Kong Stock Exchange.
In the 2023 ADC-to-BD deal, Yingen Biotech appeared in the capital market as a strong “dark horse”. At that time, the ADC assets in Yingen Biotech's R&D pipeline were all but wiped out. Following BionTech spent 1.67 billion dollars to introduce its two ADCs in April 2023, they jointly promoted the development of the third ADC drug candidate DB-1305 in August. However, when BeiGene offered a total transaction contract of 1.3 billion US dollars, it was only able to obtain a pre-clinical ADC drug. It can be seen that it is powerful.
The secondary market in 2024 will become Yingen Biotech's ambition. Recently, the company plans to obtain materials for the Hong Kong IPO to be received by the Securities Regulatory Commission. Previously, on August 26, it submitted a listing application to the main board of the Hong Kong Stock Exchange. Morgan Stanley, Jefferies Finance, and CITIC Securities were co-sponsors.
 According to the Zhitong Finance App, Yingen Biotech was founded in 2019. This innovative pharmaceutical company, which has only been in existence for 5 years, can rapidly rise and impact the capital market. It is inseparable from its founder Zhu Zhongyuan.
According to the Zhitong Finance App, Yingen Biotech was founded in 2019. This innovative pharmaceutical company, which has only been in existence for 5 years, can rapidly rise and impact the capital market. It is inseparable from its founder Zhu Zhongyuan.
Supported by the strong “circle of friends” of Zhu Zhongyuan, a former leader in the pharmaceutical investment industry, Yingen Biotech completed 4 rounds of financing in a short period of time, with a cumulative total of 0.138 billion US dollars. Investors include well-known capital such as “Pharmaceutical”, investment institutions under Eli Lilly Asia Fund, Sino-Singapore Venture Capital, Shenglian under the Seven Wolves Group, and Tianlix International Capital. After dilution, Yingen Biotech was valued at 0.27 billion US dollars, and the contract was 1.86 billion yuan (calculated at the exchange rate of September 1, 2022).
Lost more than 0.8 billion yuan after holding 12 ADC drugs for 2 years
According to the prospectus, Yingen Biotech is committed to developing next-generation ADC innovative drugs for patients with cancer and autoimmune diseases. Using its ADC technology platform, the company has established a highly innovative and differentiated pipeline composed of 12 self-developed ADC drug candidates. Specifically, six clinical-stage ADCs have potential in a wide range of unmet clinical indications. According to Frost & Sullivan, each is among the world's leaders in terms of development progress of overall or major indications; two new-generation bispecific ADCs (“bsADCs”) and an ADC for treating autoimmune diseases (“self-immune ADC”) are expected to enter the clinical phase from 2024 to 2026; and many other preclinical ADCs.
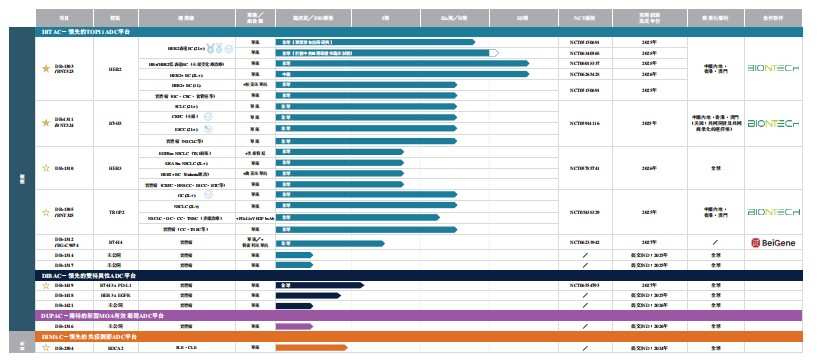
Yingen Biotech's three clinical-stage assets (including its core products DB-1303/BNT323 and DB-1311/BNT324, and key products DB-1305/BNT325) have been fast track certified by the FDA. DB-1303 has been certified as a breakthrough therapy for specific indications by the FDA and the China Drug Administration.
Yingen Biotech has also built four leading ADC technology platforms: Yingen Immunotoxin Antibody Conjugation Platform (DITAC), Yingen Innovative Bispecific Antibody Conjugation Platform (DIBAC), Yingen Immunomodulatory Antibody Coupling Platform (DIMAC), and Yingen's Unique Payload Antibody Coupling Platform (DUPAC) to break through the boundaries of ADC treatment.
Although it has 12 ADC drugs, Yingen Biotech has no commercial products on the market, and its revenue mainly depends on external licensing. Yingen Biotech revealed that the total transaction milestone value of the cooperation currently established on its ADC-related assets has exceeded 4 billion US dollars, yet the company has not broken out of the loss trap.
From 2022 to 2024 for the three months ended March 31 (hereinafter referred to as the reporting period, same below), Yingen Biotech's revenue was approximately 1.6 million yuan (unit: RMB, same below), 1.787 billion yuan, and 0.641 billion yuan respectively; losses during the period were approximately 0.387 billion yuan, 0.358 billion yuan, and 65.942 million yuan, respectively, with a total loss of approximately 0.811 billion yuan. Losses mainly stem from expenses related to R&D activities, and changes in the fair value of financial liabilities relating to preferred shares measured at fair value and the changes included in profit and loss; R&D expenses were $0.34 billion, $0.559 billion, and 0.311 billion yuan, respectively.
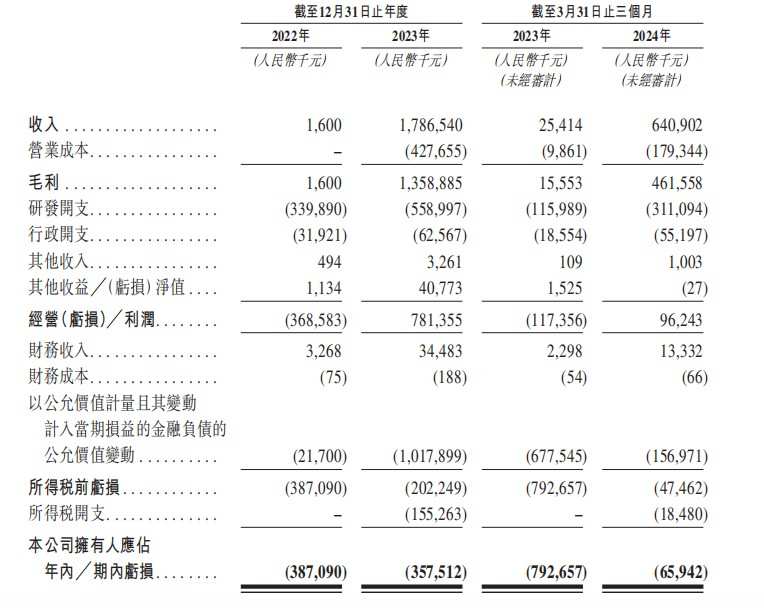
Simply put, like most biotechs, Yingen Biotech is also facing financial constraints. Even in 2023, when a large amount of licensing was achieved, Yingen Biotech still lost 0.358 billion yuan. Currently, Yingen Biotech's total assets are 1.7 billion yuan, and its total liabilities are as high as 2.8 billion yuan, making it seriously insolvent.
According to the Zhitong Finance App, the reason Yingen Biotech continues to lose money is, on the one hand, that the company needs a large amount of research and development, and on the other hand, it is due to early financing. Large investors in all rounds of pre-IPO financing mostly purchased preferred shares. As valuations increased, Yingen Biotech recorded a loss of 1.018 billion yuan due to large changes in the value of preferred shares in 2023.
Yingen Biotech also clarified the shareholders' gambling agreement in the prospectus. If specific events in the agreement cannot be reached, shareholders can claim to redeem all of their preferred shares. As of March 31, Yingen Biotech still has 1.33 billion yuan in cash on its account, and the company claims that the cash on hand is enough to cover it until March next year. Today, Yingen Biotech can no longer afford any share redemption requirements.
To this end, Yingen Biotech said that the company signed a supplementary agreement with shareholders in August to suspend the redemption function, provided that the IPO was withdrawn or rejected, or until 18 months after the initial submission date. Yingen Biotech currently has only one path to follow: seek listing on the Hong Kong Stock Exchange.
Most pipelines are in the early clinical phase and are still in the early commercialization process
In fact, choosing CXO (pharmaceutical contract outsourcing) and using BD financing for blood transfusions instead of building their own plants can be said to be the experience and survival method learned by domestic biotechs after the heat wave of the innovative drug industry in previous years. This is also more in line with Biotech's own advantages and division of labor within the industry, that is, its competitiveness lies in innovative technology research and development, while late-stage development and commercialization are the expertise of large pharmaceutical companies.
However, it should be noted that although ADC has more room for exploration as a drug type compared to previous PD-1 targets, global competition in this field is also fierce.
Looking at Yingen Biotech's pipeline layout, it has proven mature targets such as HER2 and TROP2, as well as emerging targets such as B7-H3 and HER3. Since then, it has also deployed dual-antibody ADCs (such as DB-1419) and ADCs for self-immune diseases (such as DB-2304).
In terms of progress and differentiated development strategies, take DB-1303, which targets HER2, as an example. Its first indication was HER2 expression EC, which avoided collision with marketed drugs. It is currently in the global single-arm phase 2 registration study stage, and will be submitted to the FDA (US Food and Drug Administration) for accelerated approval as early as 2025. Looking at the global market capacity of HER2 ADC, the market is expected to grow at compound annual growth rates of 30.8% and 16.8% from 2023 to 2028 and 2028 to 2032, respectively, and reach 34.5 billion US dollars in 2032.

Looking at the competitive landscape, as of the last practical date, two HER2 ADCs (i.e. Enhertu and Kadcyla) have been approved in the US, while another HER2 ADC (i.e. Edisch) has been approved in China. As of the same date, three HER2 ADCs (including Enhertu) are in phase III clinical development or later in the global multi-regional clinical trial (MRCT) phase. There are many competitors, and it can be seen that Yingen Biotech's marketization process is not too early.

In summary, although the BD transaction contributed a lot of revenue to Yingen Biotech, the company is still insolvent due to the fact that the drug has not yet been commercialized, R&D investment, and changes in the value of preferred shares brought about by early financing. Judging from the prospectus, most of the company's pipeline products are in the early clinical stages, so whether it can stand out from the fiercely competitive ADC technology platform still depends on its subsequent R&D strength and market performance.

 根据智通财经APP了解,映恩生物成立于2019年,这家成立仅5年的创新药公司,能够迅速崛起,并向资本市场发起冲击,离不开其创始人朱忠远。
根据智通财经APP了解,映恩生物成立于2019年,这家成立仅5年的创新药公司,能够迅速崛起,并向资本市场发起冲击,离不开其创始人朱忠远。