Source: a large pine tree (the-bigtree)
Author: Yang Song, head of Cinda Securities and Pharmaceutical Industry
Background:Recently, the announcement of the National centralized Drug Procurement (GY-YD2019-2) issued by the State organized Drug centralized Purchasing and use Joint Purchasing Office (Guolian Zizi (2019) No. 1, the second batch of volume procurement was officially launched.
The overall view of Cinda Securities:
Enterprises need ① to fully consider the competition situation, ② fully understands the selected rules (such as the relationship between 1.8x price difference), and the most important thing for ③ is to analyze competitors (especially this time some varieties have the participation of well-known price killers in the industry).
-- for some varieties with a more competitive pattern, especially those with a total number of bidding varieties greater than or equal to 7 (such as glimepiride), the lowest price is expected to be very low, so this kind of variety enterprises will quote the lowest price they can accept (limit cost * coefficient), and the final price drop is expected to be very large.
-- since there is a maximum price limit for this volume purchase, for the original drug research enterprises, accepting the maximum price itself means a larger reduction. On the whole, the original drug research enterprises with small price difference, domestic prices higher than international prices and strategic products are expected to have a higher probability of participating in bidding.
-- for enterprises with a large stock of varieties, if they eventually win the bid but the price reduction is too large, their profits will also be greatly affected. For some of the varieties with small stock or newly approved listing, if they can finally win the bid, according to the situation of more than half a year in the past, the consumption of most of the selected varieties has been better guaranteed to achieve price for quantity.
-- in addition to individual key varieties that need to be paid attention to, this volume procurement is expected to have little impact on the capital market as a whole. There are two main judgments. First, after nearly a year of learning about the exogenous variable of volume procurement, the enterprise and the market have a more systematic understanding and response plan, that is, the market has certain expectations for the policy itself. Secondly, at the end of 2018, some single large variety enterprises are expected to suffer a greater impact in the future, and there are more institutions, so the range of adjustment is relatively large, but the market in 2019 is mainly allocated to enterprises that are not or less affected by the impact of volume procurement.
1. Range of varieties: two metformin varieties were removed
According to the latest second batch of procurement documents, the total number of products purchased is 33. Compared with the previous version, metformin sustained-release form and metformin oral regular-release form have been removed.
Table: catalogue of purchased varieties (unit:Ten thousand tablets / ten thousand bags / ten thousand pieces) (the highest valid quotation (unit: yuan / piece, bag, branch)
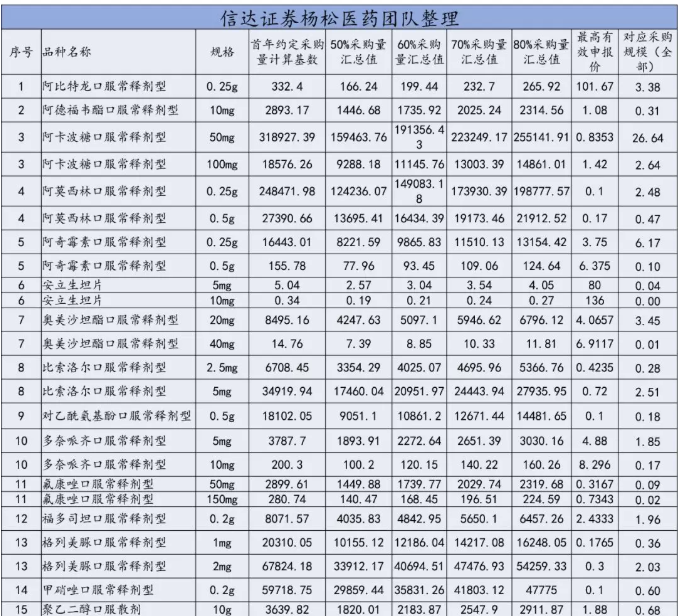
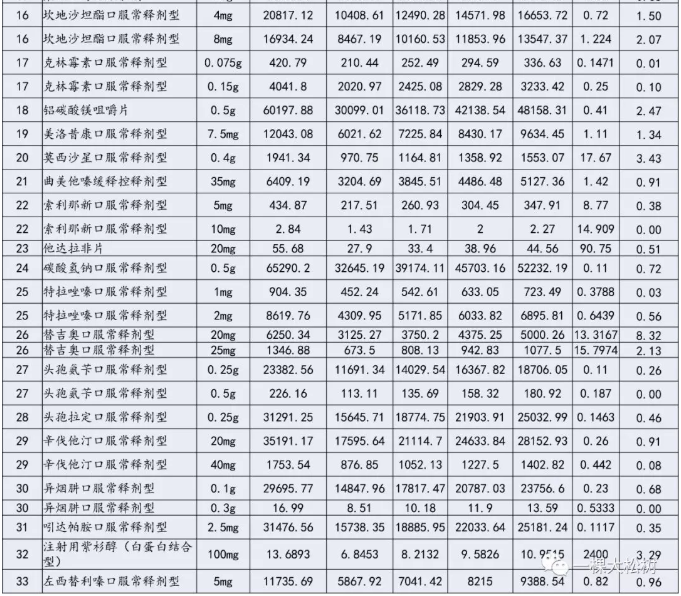
Price limit: basically all of them are below the lowest price in the country
According to the communication results of the previous meeting, the highest effective quotation will be made this time. When the enterprise declares, it is required that the "unit bid" should be less than or equal to the "highest effective bid" of the corresponding specifications of the purchased variety.
Brief comment: the rules for determining the highest valid bid are not specified in the document.Judging from the rapid retrieval of the price in the history of some varieties, the highest valid bid agreed in the document is basically based on the price determined below the current lowest winning price of the common name product in the country (some varieties may have won the bid with this price, but could not be retrieved).
Third, the overall procurement scale: about 8.8 billion as a whole.
According to the calculation base of the highest valid quotation and the agreed purchase volume in the first year, the total purchase scale corresponds to 8.8 billion, of which the largest purchase scale is 2.664 billion acarbose.
IV. Determination of purchase quantity and procurement cycle
If one enterprise is actually selected throughout the country, it shall be 50% of the calculation base of the agreed purchase volume in the first year; in principle, the current procurement cycle is one year.
If two enterprises are actually selected throughout the country, it shall be 60% of the calculation base of the agreed purchase volume in the first year; in principle, the current procurement cycle is 2 years.
If three enterprises are actually selected throughout the country, it shall be 70% of the calculation base of the agreed purchase volume in the first year; the current procurement cycle is, in principle, 2 years.
If the actual number of selected enterprises in the country is 4 or more, it shall be 80% of the calculation base of the agreed purchase volume in the first year, and the current procurement cycle is, in principle, 3 years.
-- the procurement agreement is signed every year during the procurement cycle. When renewing the procurement agreement, the agreed purchase quantity shall, in principle, be no less than the agreed purchase quantity of the selected drug in the previous year.
-- if the agreed purchase volume of the current year is completed ahead of schedule in the procurement cycle, more than some of the selected enterprises will still supply at the selected price until the expiration of the procurement cycle.
Brief comments:The design of the rules here is more reasonable, fully taking into account the pattern of market competition, for more competitive varieties, by extending the procurement cycle, we can better protect the supply of drugs.
The scope of declared varieties: overrated varieties + original research (reference preparation)
Listed drugs that fall within the scope of the catalogue of purchased varieties and have obtained valid registration documents in China.
And meet one of the following requirements:
The main results are as follows: 1) the reference preparation for evaluating the consistency of the quality and efficacy of generic drugs issued by the original drug and the SDA.
2) generic drugs which are evaluated by the quality and efficacy consistency of generic drugs by the State Drug Administration.
3) generic drugs approved according to the newly registered classification of chemicals according to the notice of the State Food and Drug Administration on the publication of the work plan for the reform of the registration and classification of chemicals (No. 51 of 2016).
4) drugs included in the catalogue of drugs listed in China
Brief comments:It should be noted that the above four items belong to the juxtaposition conditions, that is, they meet one of them. Some of the varieties discussed in the industry before have not been found in the listed drug catalogue, but as long as the varieties meet the newly registered classification, you can participate in this with-volume purchase.
6. agreement on the use and usage of the selected products: priority is required, and supporting rules are expected to be issued in various places.
The plan requires that during the procurement cycle, medical institutions will give priority to the use of the selected drugs in this centralized procurement and ensure that the agreed purchase volume is completed. On the basis of giving priority to the use of the selected drugs in this centralized drug procurement, the remaining dosage can be purchased appropriately in accordance with the relevant provisions of the local centralized drug procurement management, and other drugs of the same variety at a suitable price.
Brief comments:It is expected that the subsequent provinces will, in the light of the actual situation, issue relevant documents on purchase and sales agreements, drug distribution, quality testing, price adjustment of unselected drugs, health insurance payment standards, and so on.
VII. Rules for successful selection: three conditions
1. The number of shortlisted enterprises is determined according to the actual number of enterprises declared: up to 6 shortlisted enterprises

2. Price conversion and ranking
According to the drug price difference rules, the price converted to single tablet / single bag / single dose, etc., is regarded as the "unit comparable price" (that is, the minimum specification price), and the order of shortlist and supply area confirmation is determined according to the price from low to high.3. Successful selection rules
If the price of the shortlisted enterprise meets one of the following conditions, it will be eligible for the proposed selection:
1) "unit comparable price" ≤ is 1.8 times of the lowest "unit comparable price" of the same variety.
2) "unit quotation" decreased by ≥ 50.00% (corresponding to "Purchasing Variety Catalog"The highest valid quotation for specifications shall be calculated on the basis of the base.
3) the "unit comparable price" ≤ is 0.1000 yuan.
Brief comments:The rule here is more critical, so let's make it clear that there is also a juxtaposition condition here, that is, one of them can be satisfied. Among them, the first article is 1.8 times, that is, the circuit breaker mechanism proposed in the previous meeting communication, that is, the maximum price shall not be higher than 1.8 times the lowest price. The second is the requirement for a reduction, that is, to avoid vicious competition, that is, if an enterprise drops by more than 50%, it can be selected regardless of the situation of its competitors. The third is to consider the extreme price situation to prevent the absolute price from being too low (such as 6 cents of amlodipine).
The main considerations for personal understanding of the design of this rule are: first, make full use of the market competition of enterprises, it is easy to be cut off by offering high prices, secondly, enterprises are encouraged to lock in the selection by 50% price reduction, and finally, enterprises are encouraged to explore limit supply prices.
VIII. Province selection: the lowest price gives priority to 2 provinces.
According to the rules, the first priority enterprise will give priority to selecting one of the supply areas. After the priority selection is completed, starting from the first order enterprise, all the enterprises to be selected will alternately confirm the remaining areas in order, and each enterprise to be selected will select one region at a time, and repeat the above process until the selection of all regions has been confirmed.
Brief comments:Under the rule here, that is, enterprises with the lowest price can first choose two provinces, which will have an impact on the quotation strategy of some products with more concentrated sales.
IX. Time and place of bid opening
(1) time: 10:00, Friday, January 17, 2020
(2) location: first floor, Building 6, 1800 Tianshan Road, Changning District, Shanghai
X. announcement of the result
The document is not clear, according to the previous first batch of volume purchase, it is expected to be about a week.
Brief comments:This year's New year's Eve is January 24. According to this pace, the final result should have been given before.A Happy New Year to one and all!
Edit / Iris
